Non-Invasive CGMs: Are We There Yet?
Nov 06, 2025
Every few weeks, a new headline promises the first truly non-invasive continuous glucose monitor (CGM) that will end finger-sticks and sensor insertions forever.
The dream is seductive: a painless, disposable-free glucose tracker built into a smartwatch or patch.
But is it real?
Get Access To Updated Diabetes Technology Courses
The Promise and the Problem
The idea of measuring glucose without penetrating the skin has fascinated researchers for more than 40 years.
It sounds simple: shine light, collect sweat, or read subtle electrical or thermal signals that correlate with glucose.
In practice, the challenge is brutal.
Glucose concentrations in interstitial fluid are tiny.
Skin, fat, and water distort optical signals.
Motion, temperature, and hydration add noise.
The result? A long list of companies that promised “no more finger-sticks” and quietly disappeared.
According to The Pursuit of Noninvasive Glucose (9th Edition, 2023), more than 200 different groups have pursued this goal since the 1980s.
Only a handful remain.
The New Wave (2025)
Despite the odds, interest has never been higher.
Around 37 companies are still actively developing or marketing “non-invasive” or “minimally invasive” CGM-like devices.
Here’s who’s still in the game:
Microneedle-based CGMs (technically minimally invasive CGMs)

- Biolinq (US) – Shine patch; FDA-cleared as a glucose-range sensor for the forearm, for adults >22 years with type 2 diabetes not using insulin.
- Sava (UK) - SAVA CGM; upper-arm microneedle patch.
- Sensible (Netherlands) – idi; upper-arm microneedle patch.
- One Heath Biosensing (US) – One Health Biosensor; upper-arm microneedle patch.
Fluid Extraction & Reverse Iontophoresis

- Nemaura Medical (UK) – SugarBEAT / ProBEAT; CE-marked adhesive patch using gentle current to extract interstitial fluid (= reverse iontophoresis).
- GlucoModicum (Finland) – Talisman; patch using magnetohydrodynamic extraction of interstitial fluid.
Sweat-Based Sensors

- Perspirion (US) – wearable fingertip hydrogel platform measuring glucose and electrolytes in perspiration.
- Onalabs (Spain) – ONADM; patch sensor placed on the back, measuring glucose and electrolytes in sweat.
Raman Spectroscopy (light scattering signature of glucose)
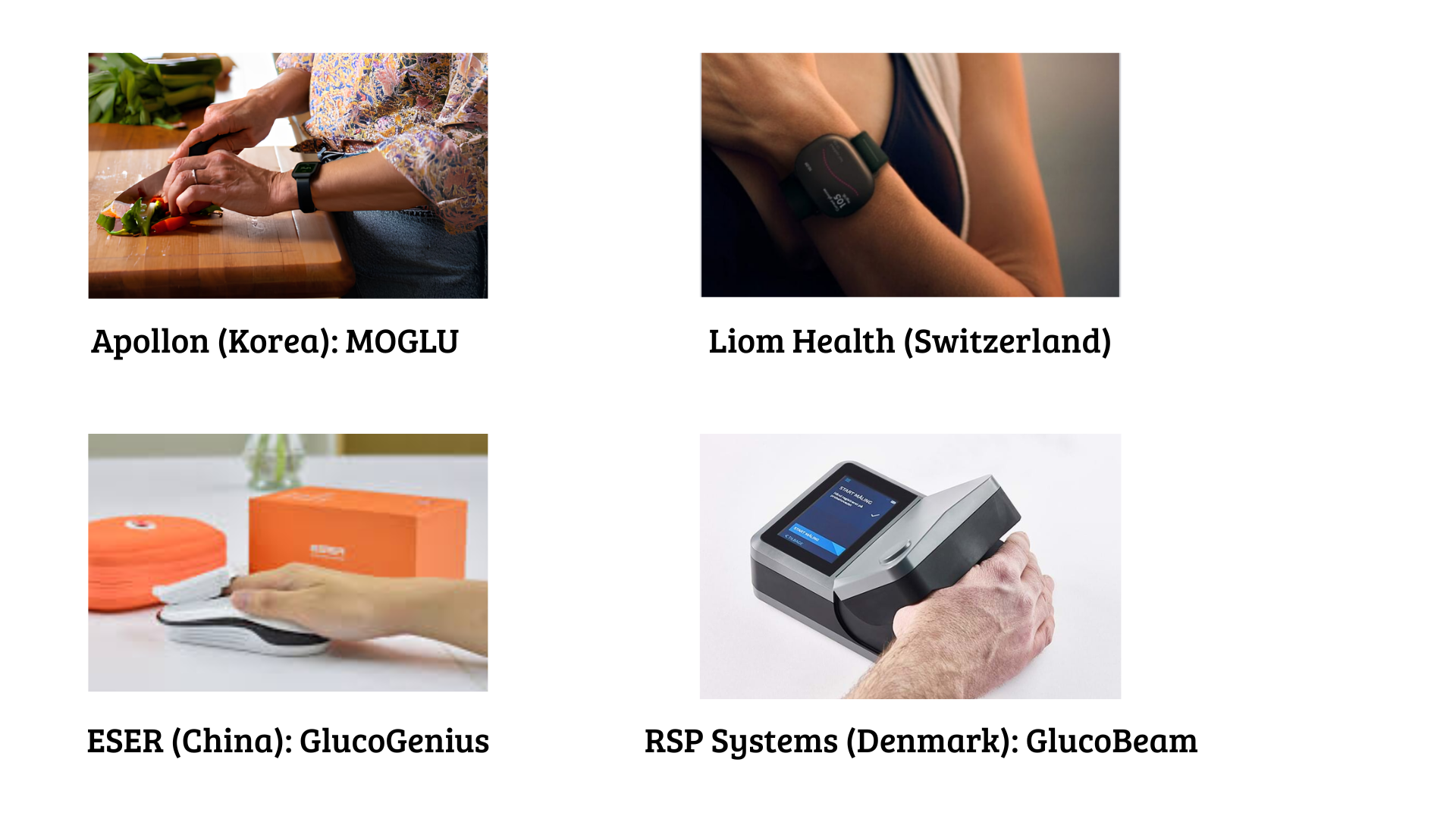
- Apollon (Korea) – MOGLU wristband.
- Liom Health (Switzerland) – wrist device in testing.
- ESER (China) – GlucoGenius; finger sensor.
- RSP Systems (Denmark) – GlucoBeam; finger sensor in clinical evaluation.
Radiofrequency / Impedance
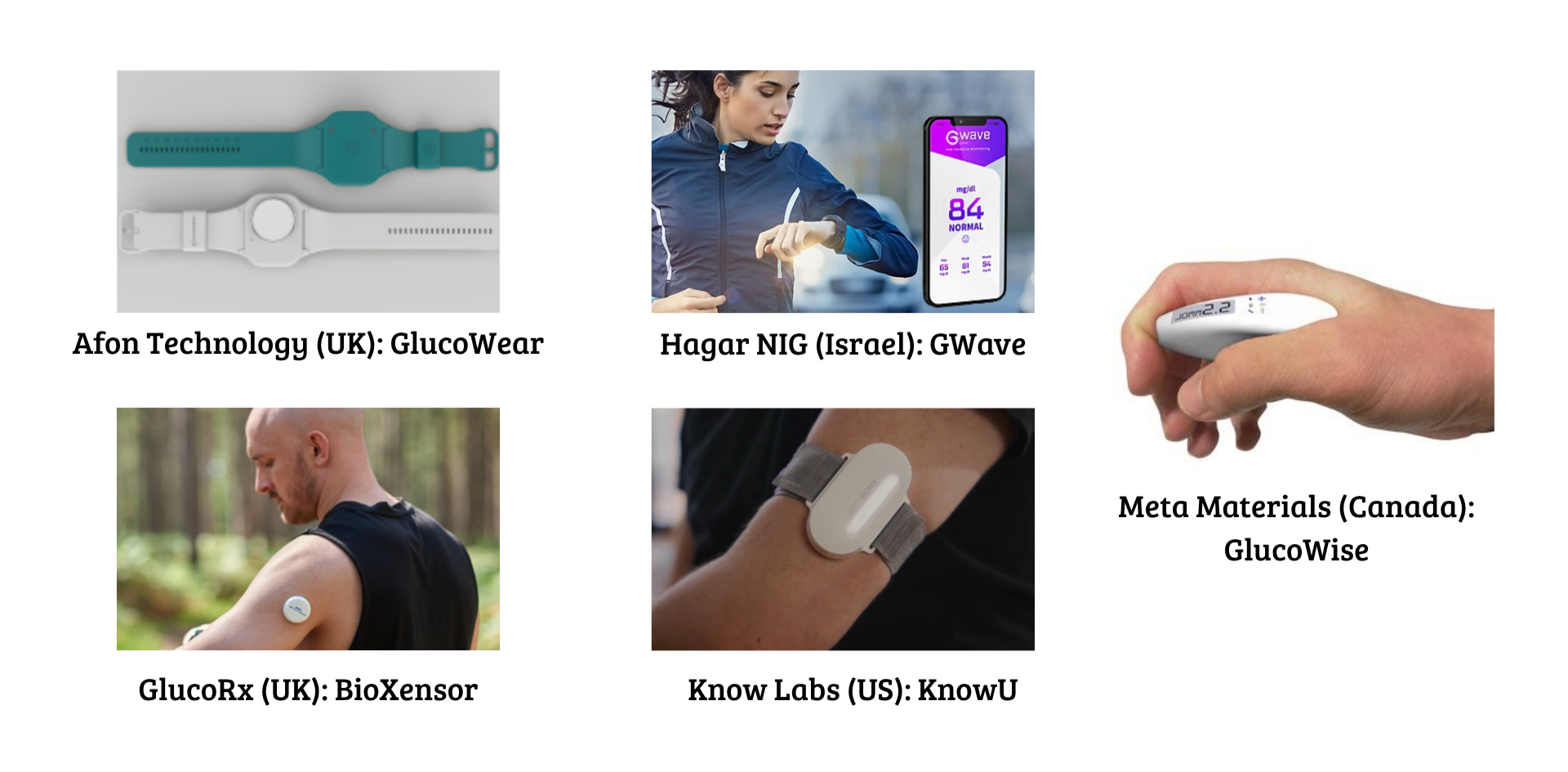
- Afon Technology (UK) – Glucowear; wristband.
- HAGAR NIG (Israel) – GWave; wrist device.
- GlucoRx (UK) - BioXensor; upper arm sensor.
- Know Labs (US) – KnowU; portable wrist or forearm sensor.
- META Materials (Canada) – GlucoWISE; handheld concept.
Join our free courses on CGMs, pumps, and AID systems
Photoacoustic & Mid-Infrared Spectroscopy

- Eclypia (France) – NeoGly; AI-enabled mid-IR photoacoustic sensor (wristband).
- DiaMonTech (Germany) – D-Pocket (fingerclip) & D-Sensor (wristband) using mid-IR photoacoustic spectroscopy.
Near-Infrared Spectroscopy

- Woori IO (Korea) – E-GluCheck; finger sensor.
- iGluco Tech (Spain) – Glucube; fingertip sensor.
- CNOGA (Israel) – CoG; finger-clip system (CE label) + capsule.
- Light Touch Technology (Japan) – mid-IR fingertip sensor.
- Biopop (US) – Elixir; hand scanner.
- GlucoActive (Poland) – GlucoStation; forearm device.
Emerging / Hybrid Concepts

- NIQS Tech (UK) – quantum glass biosensor.
- Synex (Canada) – MRI-based fingertip spectroscopy.
- Ambrosia (US) – Rizz; optical + thermal + motion sensing.
- LifePlus (US) – LifeLeaf; machine-learning-enabled PPG wristband.
- VitaSensor (US) - PPG & semiconductor based wristband and fingertip sensor
- MYMONX (UK) – MXW1/MXW2; PPG-based smartwatch concept.

- BlueSemi (India) - Eyva; AI-enabled fingertip sensor.
- PreEvt (US) – Isaac; breath acetone sensor to detect hyperglycemia via a clip.
- BOYDSense (France) – breath Volatile Organic Compounds (VOC) detection.
- SynchNeuro (US) – EEG-based behind-the-ear sensor.
- Occuity (UK) – Indigo; pen-sized eye-scan.
- Diawiser (Lithuania) - AI-powered voice analysis.
(Only commercial projects with public updates in the past two years are listed.)
Why It’s Still So Hard
The main obstacles haven’t changed in four decades:
- Signal-to-noise – Glucose’s optical signature is faint and easily drowned by water and tissue.
- Specificity – Hydration, temperature, and perfusion changes imitate glucose variation.
- Calibration – Every person’s tissue optics differ; universal algorithms rarely hold.
- Regulation – FDA accuracy thresholds (±15 mg/dL) are unforgiving, and few devices pass consistent human trials.
The New Approval Landscape: A Subtle Shift

Biolinq’s clearance marks a notable milestone.
Though not a full CGM, it is the first FDA-cleared glucose-range sensor
— a device that shows whether glucose is in range or out of range rather than providing numeric values or trend arrows.
This could signal a regulatory shift:
an intermediate class between wellness trackers and medical-grade CGMs.
Still, its clinical utility is uncertain.
One of the main benefits of CGM lies in trend awareness and behavioral feedback.
A range-only sensor omits those insights,
though it may still help people with type 2 diabetes not using insulin, for whom the aim is awareness rather than dosing guidance.
The Silent Struggle of Tech Giants
That even Apple, Samsung, and Google have not yet managed to bring a non-invasive glucose monitor to market says it all.
- Apple has been working for over a decade on optical glucose sensing within its “Exploratory Design Group.”
- Samsung stated in 2024 that it hopes to bring non-invasive glucose monitoring to market “within five years.”
- Google Verily abandoned its glucose-sensing contact-lens project in 2018 after tests showed “insufficient consistency between glucose in tears and blood.”
Even with vast resources, none of these projects has achieved clinical validation.
Growing Warnings
In February 2024, the FDA issued a clear statement: no smartwatch or smart ring that measures blood glucose non-invasively has ever been authorized.
The agency cautioned that inaccurate readings could cause dangerous insulin-dosing errors leading to confusion, coma, or death within hours.
Belgian authorities recently echoed this concern. The Federal Agency for Medicines and Health Products (FAMHP) warned that non-invasive glucose meters are unreliable and potentially dangerous for people with diabetes.
Together, these warnings have effectively paused the consumer race, underscoring that sensor accuracy — not design — remains the decisive factor.
The Author’s Verdict

John L. Smith’s The Pursuit of Noninvasive Glucose ends bluntly:
“Virtually all past efforts have failed commercially, and no fully validated, clinically approved non-invasive continuous glucose monitor exists yet.”
Even today, most surviving companies are still at prototype or wellness-device stage.
But two dynamics have changed:
- Technology – AI-driven signal correction and miniaturized photonics are improving.
- Regulation – The FDA’s recognition of a “glucose-range” category could open a door to simplified non-invasive monitoring.
So, Are We There Yet?
Not yet — but closer than ever.
The convergence of machine learning, optical physics, and wearable design is narrowing the gap.
The next breakthrough may not look like today’s CGM — it might be simpler, subtler, and aimed at awareness rather than dosing.
Until then, non-invasive glucose monitoring remains what it has always been:
the holy grail of diabetes technology
— almost here, never quite arrived.
📘 Read More
For those interested in the complete history of this fascinating and often frustrating pursuit,
the full 9th Edition of John L. Smith’s The Pursuit of Noninvasive Glucose: “Hunting the Deceitful Turkey” can be freely downloaded here:
👉 Download The Pursuit of Noninvasive Glucose (9th Edition, 2023)
This seminal work is no longer hosted on the author’s website, but with his permission, Diabetotech is proud to preserve and share it
— an essential, honest chronicle of forty years of innovation, failure, and enduring hope in the search for needle-free glucose monitoring.
Kind regards,