EASD 2025 in Vienna – Technology Highlights
Oct 01, 2025
The EASD 2025 in Vienna was a major success!
More than 13,500 people attended the congress, which featured over 1,900 presentations across 4.5 days: 52 scientific symposia, 50 industry symposia, 23 NGO/study group symposia, and 192 Oral and Short Oral sessions (the latter already published in Diabetologia by late August 2025).

The EASD (European Association for the Study of Diabetes) traditionally emphasizes both clinical and fundamental diabetes research. While pharmacological treatment has usually dominated, this year there was significantly more attention for technology.
Here are my personal technology highlights from EASD 2025:
Get Access To Updated Diabetes Technology Courses
CGMs from Asia on the rise
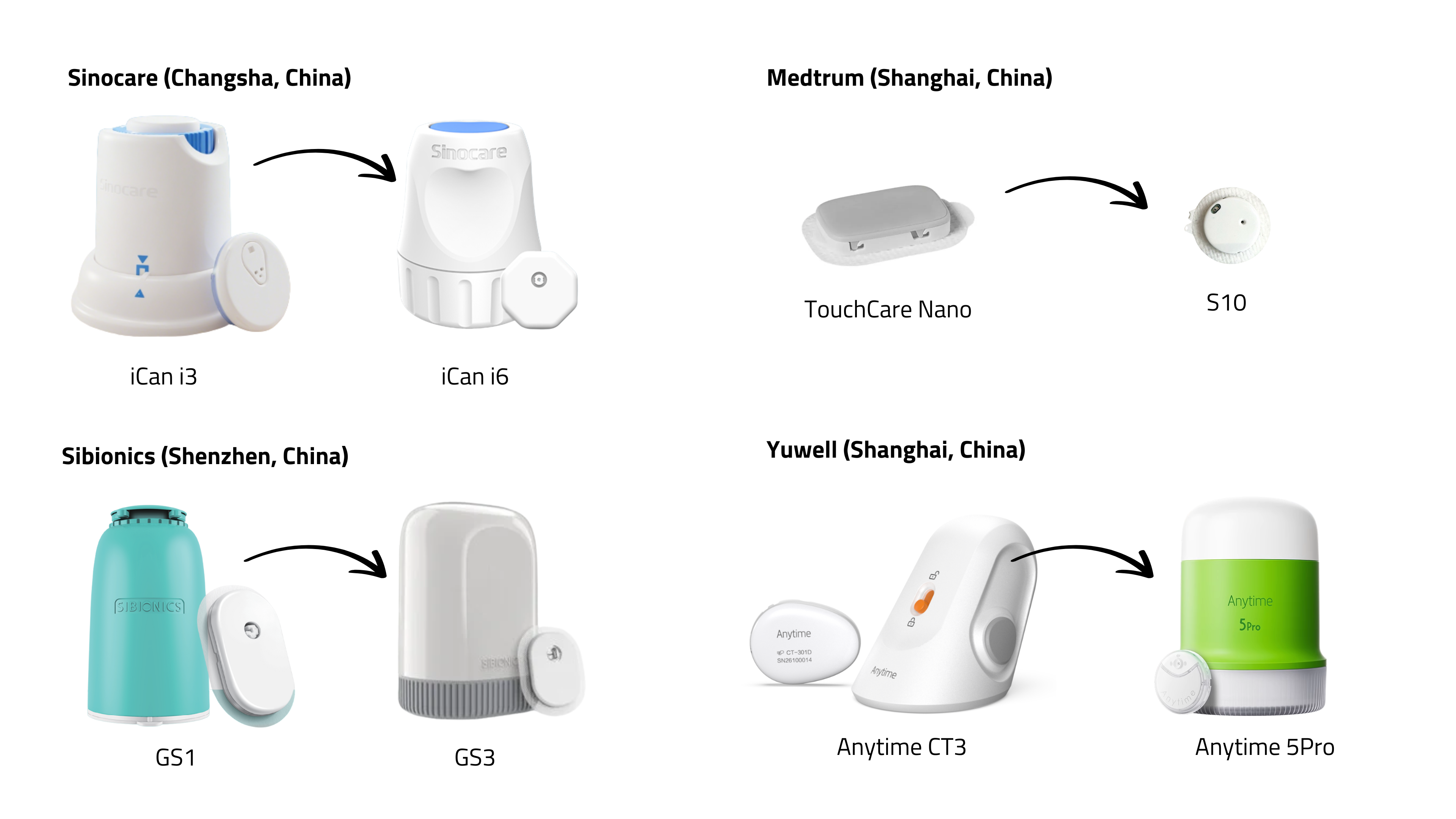
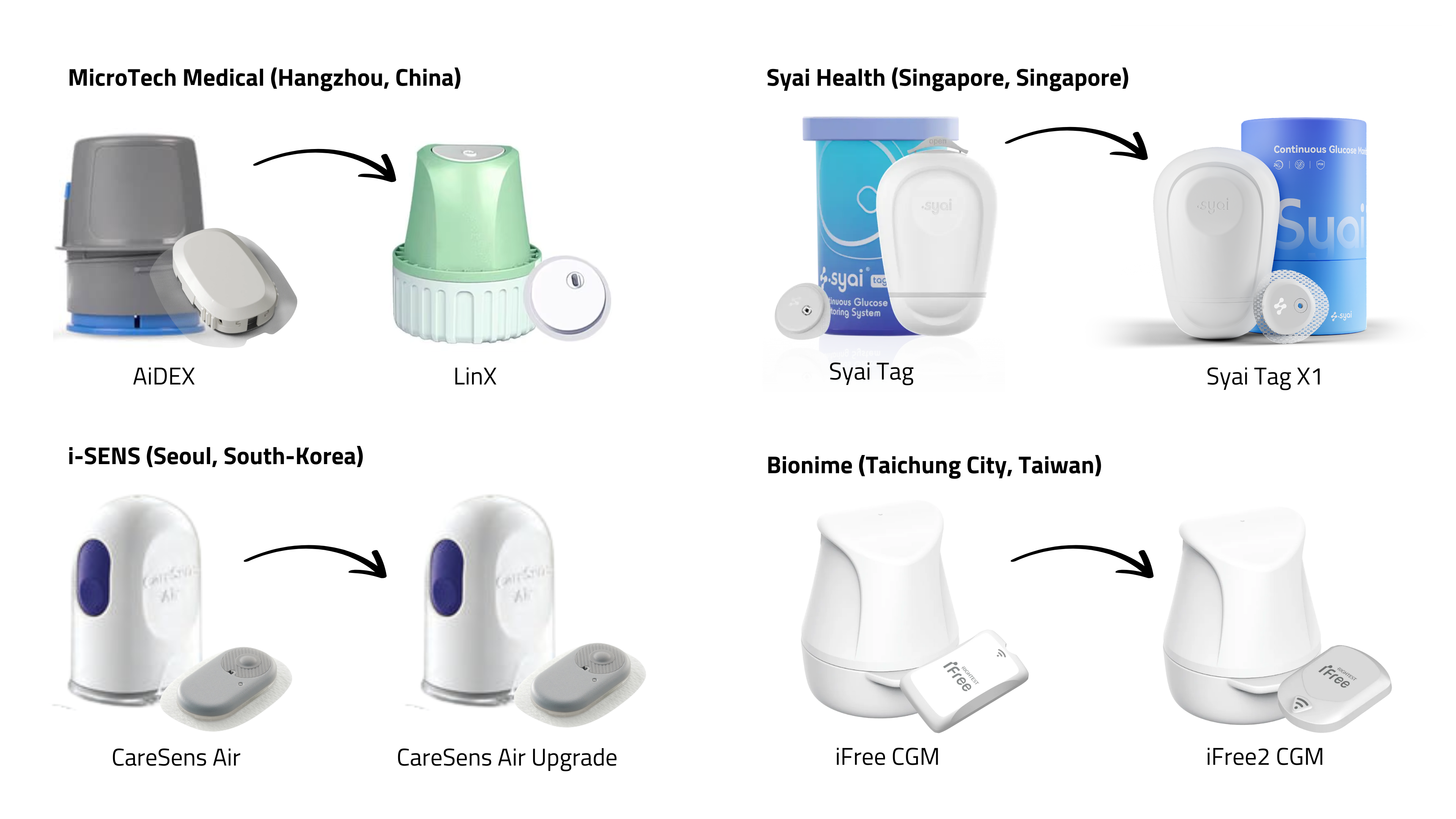
A wave of new CGMs from Asia is in the pipeline: iCan i6 (Sinocare), GS3 (Sibionics), LinX (MicroTech Medical), S10 (Medtrum), Anytime 5Pro (Yuwell), Syai Tag (Syai Health), CareSens Air Upgrade (i-Sens), and iFree 2 (Bionime).
The exhibition hall showcased this next generation—smaller, lighter, flatter, mostly white or grey, with integrated sensors and applicators. Most:
- last 15 days,
- require no fingersticks (but allow calibration),
- claim MARD <10%,
- already hold CE-marking (although none are FDA-cleared),
- are being reimbursed in some EU countries,
- are marketed through well-known distributors (e.g. Menarini, Mediq, GD Medical).
Noticeably absent was Glunovo CGM (Infinovo), which already has a CE-marked P3 CGM in Europe.
Positive accuracy data were presented for the GS3 and iCan i3.
However, Sinocare has withdrawn its FDA submission for the iCan i3 to focus on the newer iCan i6, which better aligns with evolving regulatory requirements.
At EASD, discussions centered primarily on the need for stricter international standards on CGM accuracy.
Key concerns:
-
Current study criteria are too lenient.
-
Reference values differ (capillary vs. venous).
-
Too few requirements exist for testing CGMs during rapid glucose rises or falls.
-
Without uniform protocols, metrics such as time in range (TIR) are not comparable across devices.
Why this matters:
Uniform international standards for CGMs are still lacking. As a result, reliable diagnostic thresholds and treatment targets for people with early-stage diabetes are difficult to establish. It also remains nearly impossible to compare devices directly.
A recent Diabetes Care letter therefore calls for the development of international accuracy standards for CGMs.
This call was echoed in a recent FIND webinar, supported by a factsheet.
In addition, DTN-UK publishes a bi-monthly updated comparison chart of CGM accuracy and study design.
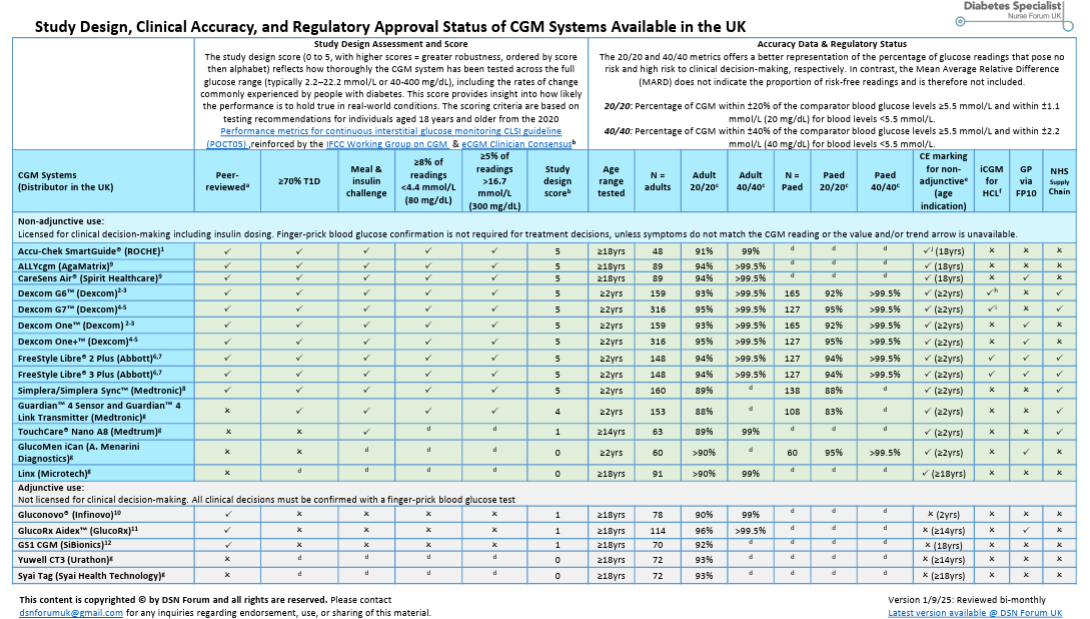
For a clear and accessible explanation of this issue, see John Pemberton’s blog.
Other CGM updates
- Roche announced that the Accu-Chek SmartGuide CGM will soon be integrated into the mySugr app.
- Dexcom introduced its Smart Basal feature, which is now under FDA and CE mark review.

CGM in gestational diabetes
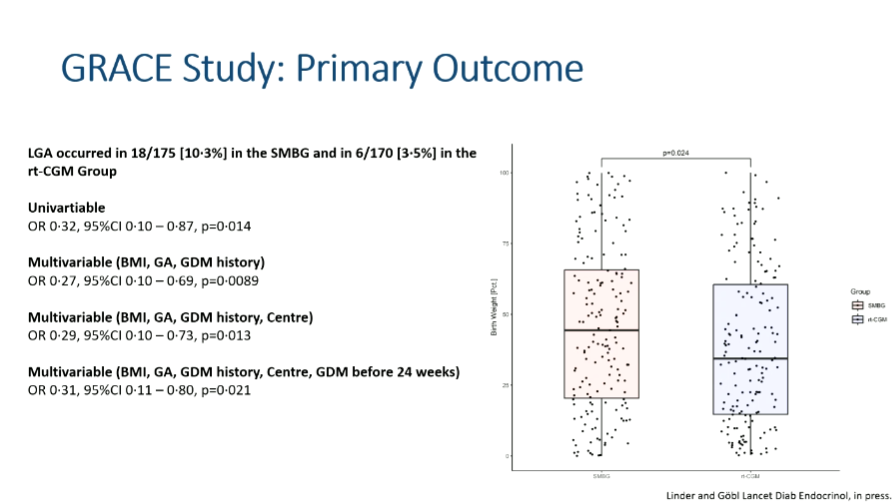
Two large randomized studies from Europe delivered partly conflicting results:
- GRACE study (n=375; Austria, Germany, Switzerland):
Women using rtCGM had fewer large-for-gestational-age (LGA) babies (3.5% vs. 10.3%), lower birthweight percentiles, and slightly more time in the strict pregnancy range (63–140 mg/dl). On the other hand, there were also more small-for-gestational-age infants, and some noted concern that the LGA rate in the CGM group was even lower than in the general population (~10%). (Abstract available; full paper pending.) - DipGluMo study (n=299; Switzerland):
No difference at all—10% LGA in both groups. Explanations may include healthier baseline cohort, less insulin use, and more flexible treatment algorithms.
Interpretation: CGM is valued by women with gestational diabetes and can improve glucose control. However, impact on outcomes is mixed. It may reduce macrosomia risk in some settings but risks overtreatment in others. Guidelines will need to balance patient preference, feasibility, and the risk of both oversized and undersized babies.
CGM during IV insulin infusion in Diabetic KetoAcidosis (DKA)
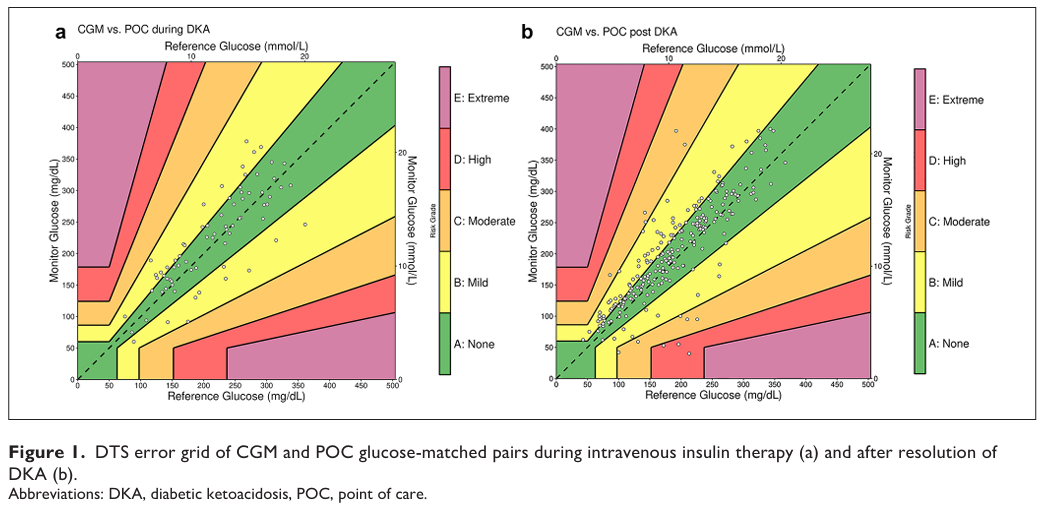
Traditionally, CGM is avoided during IV insulin infusion because of concerns about accuracy. Instead, frequent fingersticks are used.
A single-center pilot study from Columbia University explored the use of Dexcom G6 in 52 adults hospitalized with DKA.
CGM was initiated once glucose levels dropped below 350 mg/dl.
Key findings:
- MARD ~17–20%, with 95–100% of values in Clarke/DTS error grid zones A/B.
- Fingerstick frequency was reduced.
- Clinical outcomes (time to DKA resolution, hospital stay) were similar to standard care.
- Patients were managed safely outside the ICU under protocol.
Conclusion: This was the first prospective report on CGM use during IV insulin in DKA. It suggests CGM could be feasible and may reduce monitoring burden, but results are preliminary. Larger multicenter trials are required before changing practice.
MiniMed 780G in people on dialysis
Prof. Nørgaard discussed the use of closed-loop systems—particularly the MiniMed 780G—in people with type 1 or type 2 diabetes and advanced chronic kidney disease, including those undergoing dialysis. This group represents a triple challenge: insulin resistance often drives hyperglycemia, prolonged half-life of insulin and impaired counter-regulation increasing the risk of hypoglycemia.
A short crossover study in Australia in 30 people with advanced chronic kidney disease showed that the MiniMed 780G achieved a TIR of 71% compared to 55% on MDI, without significant increases in hypoglycemia. (Abstract en Poster available)
Practical cases presented:
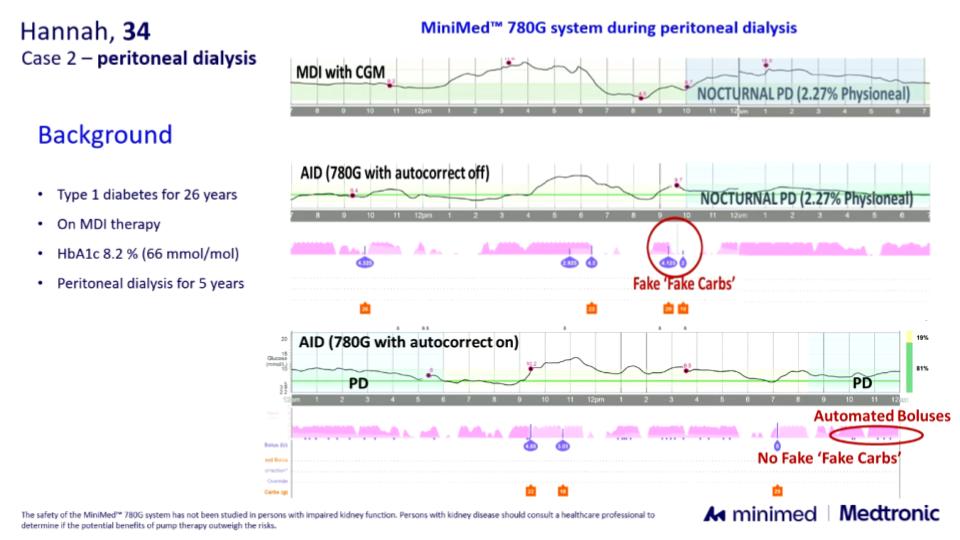
- Peritoneal dialysis: despite the glucose-rich dialysate, autocorrections in the MiniMed 780G managed the expected glucose rises effectively. In some cases, a small amount of “false carbohydrates” was needed beforehand, but overall regulation remained stable.
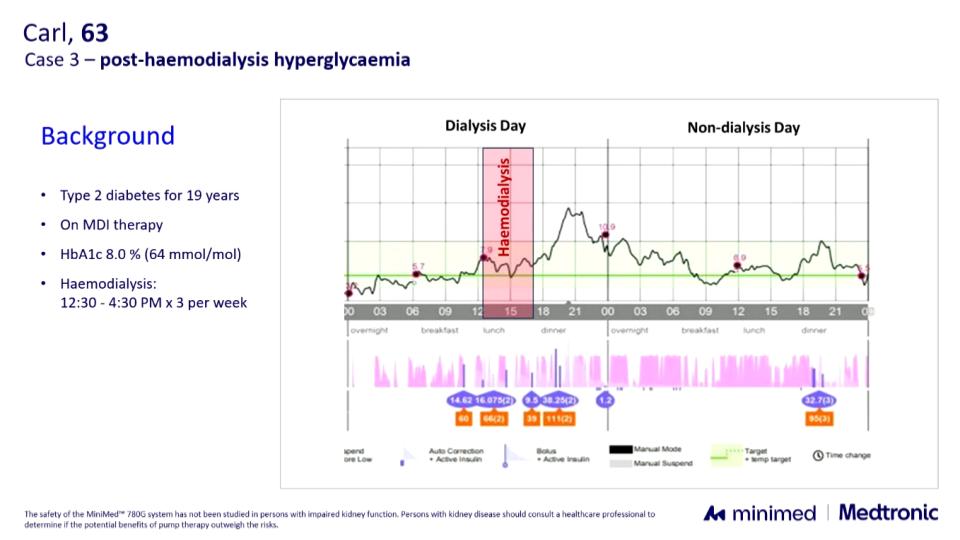
- Hemodialysis: in one patient with frequent post-dialysis hyperglycemia, automode normalized glucose within a few hours.
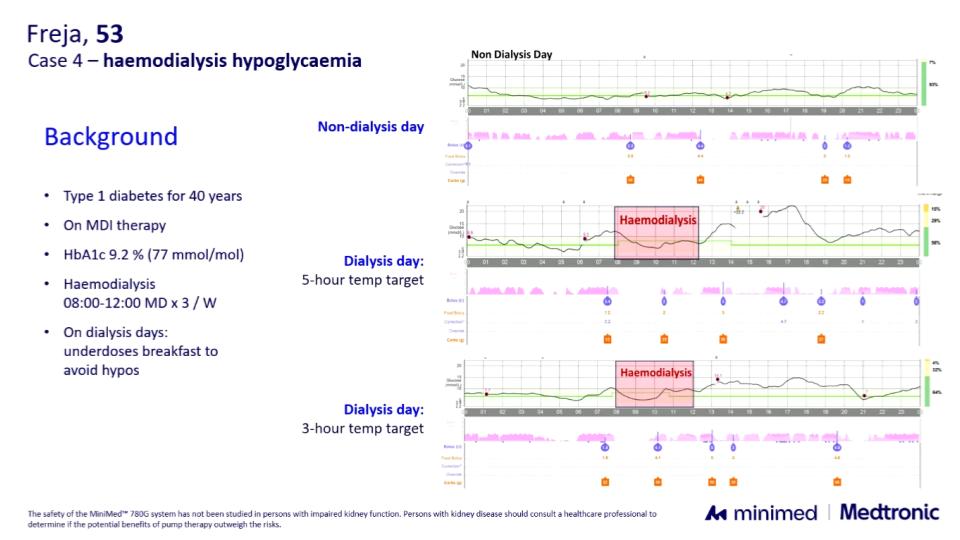
- In another case with repeated dialysis-related hypoglycemia, a temporary target was useful during dialysis. A duration of 5 hours led to hyperglycemia, but adjusting to 3 hours—set at the start of dialysis—provided good results.
Conclusion: Although still off-label, these data and real-world examples suggest that the MiniMed 780G can be both safe and effective in dialysis. They provide not only reassurance but also concrete tips for fine-tuning closed-loop use in this particularly complex population.
These insights are reinforced by a recent London multicenter case series, where nine adults with type 1 diabetes on hemodialysis using various AID systems (including 780G) improved TIR (39.7% → 59.8%), HbA1c (78.6 → 56.1 mmol/mol), and dialysis-day TIR (32.7% → 62.3%) over 7 months, with no severe hypoglycemia or DKA.
Cost-effectiveness of AID systems in the Nordics
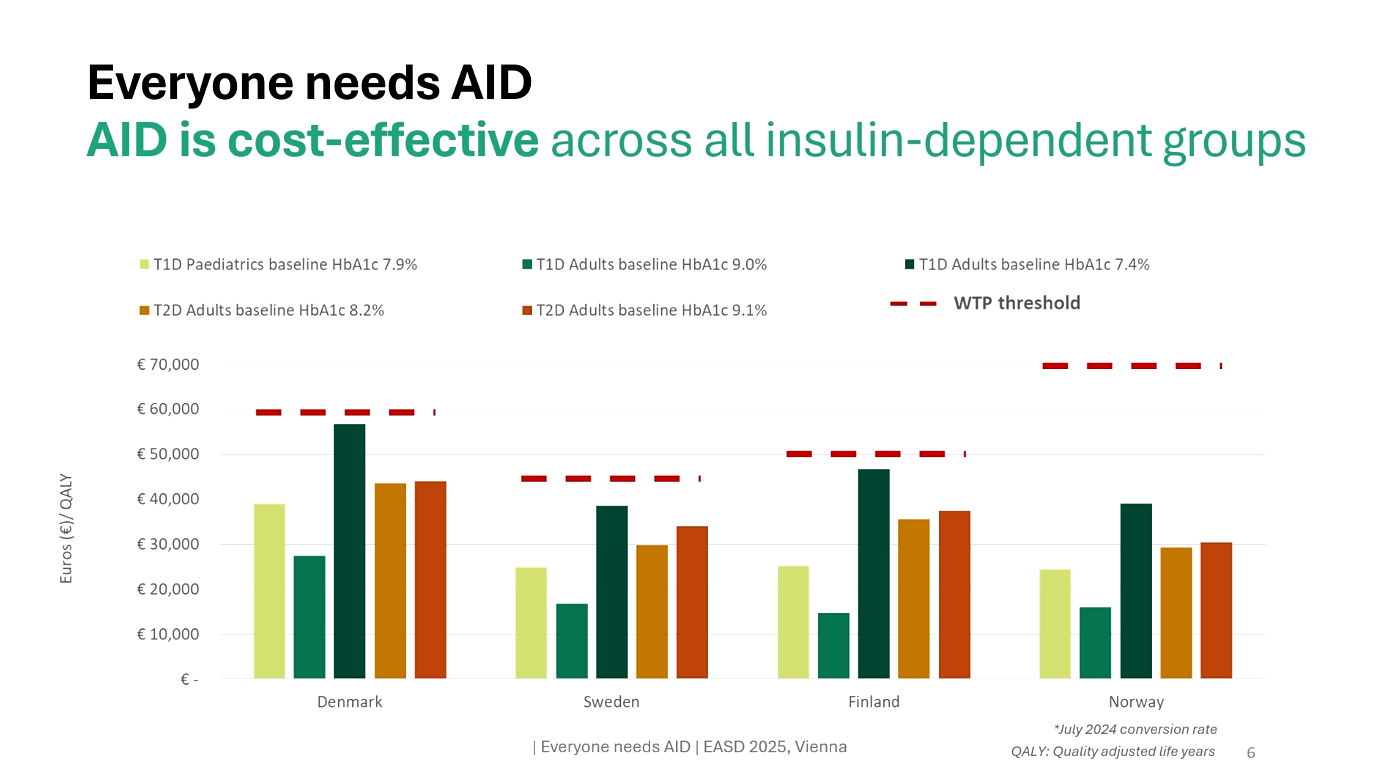
Parallel to the clinical data, a health-economic analysis modeled AID vs MDI+CGM over 30 years in Sweden, Norway, Denmark, and Finland using the IQVIA Core Diabetes Model. (Abstract & poster available)
- HbA1c reduction assumptions: −0.7% (pediatric T1D), −1.4% (adult T1D), −1.3% (adult T2D).
- Outcomes: QALY gains in all groups (1.4–1.5 in T1D, 0.8 in T2D), 23–48% fewer complications, and ICERs below willingness-to-pay thresholds in all countries.
Poster conclusion: AID therapy leads to both short- and long-term clinical benefits alongside reduced economic impact. In the Nordic region, AID has been shown to be cost-effective across all analyzed groups. Enabling proper and timely access to AID for insulin-dependent patients could provide significant benefits for patients, caregivers, healthcare systems, and society as a whole.
New guidelines in the making

Three upcoming consensus reports were presented in draft form:
- 2026 ADA/EASD Type 1 Diabetes treatment guideline (open for comment).
- International consensus on diabetes technology for pregnant women with type 1 diabetes, type 2 diabetes & gestational diabetes (DiaTribe-led).
- Consensus on continuous ketone monitoring (update of 2021).
All emphasize that technology is becoming central in future diabetes care.
We look forward to reporting on these consensus statements in the future. :-)
Take-home
EASD 2025 showcased how rapidly diabetes technology is evolving—from CGMs from Asia to closed-loop systems in dialysis and even early steps toward continuous ketone monitoring.
At the same time, stricter regulation, standardized accuracy, and nuanced clinical interpretation remain essential. Progress and caution must go hand in hand.
As Carl Jung put it:
“Only the paradox comes anywhere near to comprehending the fullness of life.”
In diabetes technology too, progress and restraint walk side by side. The real challenge is to strike the balance—so that innovation truly translates into safer, better care for people with diabetes.
For this blog I focused only on the technology highlights. Of course, EASD 2025 was much broader in scope, with GLP-1 receptor agonists and related therapies again taking center stage in many sessions.
Kind regards,