Quarterly Update Diabetes Technology
Nov 30, 2022
It's that time of year again: the leaves are turning yellow and red, the days are getting shorter and colder, and the listed companies are giving out their quarterly update for their investors!
If you're a healthcare professional or someone with diabetes, you know how important it is to stay on top of the latest diabetes technology.
In this post, we take a look at some of the highlights from the latest investor calls from relevant companies.
So sit back, relax and get ready to learn all about the latest advancements in diabetes technology!
Get Access To Updated Diabetes Technology Courses
- 1. Tandem
- t:connect Mobile app submitted for CE label
- Mobi insulin pump submitted for FDA label
- 7 days infusion set in the pipeline
- "Higher IQ" = Control IQ for people with an insulin need >100 U/d
- 2. Insulet
- New basal-only Omnipod
- Costly PDM recall
- 3. Senseonics
- Update Gemini project
- 4. Dexcom
- Dexcom G7 resubmitted for FDA label
- Dexcom ONE will also evolve to Dexcom G7 format
- 5. Abbott
- Abbott is and will remain the largest
- Lingo will launch in 2023
- Novopen 6 integrated into Libreview
- mylife Loop with Libre3 has been launched
- 6. Eoflow
- Update pipeline Eoflow!
Read on below for more details!
(PS: Medtronic also had an investor call on 11-22-2022, but most of it has already been mentioned in previous blogs.)
1. TANDEM
The following news comes from the 11/2/2022 (3Q22) investor call.
Click here for extensive press release & audio.
*t:connect Mobile app submitted for CE label

The t:connect app not only allows you to view your pump data on your mobile phone, but also to give an insulin bolus from your phone.
It is currently available in the US for compatible Android and iOS mobile phones.
Tandem has now -finally- applied for a CE label for the t:connect mobile app.
*Tandem - Mobi pump submitted for FDA label

The Mobi pump is 50% smaller than the Tandem t:slim X2 pump and is controlled from your mobile phone.
This pump was announced last year, and Tandem filed for FDA approval this quarter.
They hope to market the Mobipump in 2023.
*Tandem - 7 days infusion set in the pipeline
Tandem purchased Capillary Biomedical in July 2022 with the goal of marketing their 7-day infusion set (SteadiFlow).
They will soon start "pivotal trials" in order to apply for an FDA label.
*Tandem - Higher IQ = Control IQ for people with an insulin need >100 U/d
Tandem Control IQ is currently only indicated in people who use <100 U of insulin per day.
They are also now conducting a study to evaluate Control IQ in people with type 1 diabetes (DM1) who need more insulin, in order to expand the indication.
2. INSULET
The following news comes from the 11/3/2022 (3Q22) investor call.
Click here for extensive press release & audio.
*New basal-only Omnipod
Insulet has submitted an application to the FDA for a newly developed basal-only Omnipod pump.
This patch pump is intended for people with type 2 diabetes (DM2) who use only basal insulin. There will be no connection to a CGM.
They hope to launch this version of the Omnipod in 2024.
*Costly PDM recall
The PDMs (Omnipod's remote control or "Personal Diabetes Manager") of the Omnipod DASH would be at risk of
- swelling of the battery,
- leakage
- or extreme overheating.
Insulet would have received about 50 complaints, including 3 fires.
They then voluntarily decided to replace all PDMs, both the Omnipod DASH and the Omnipod 5.
This will cost them about $37 million...
For those who still use the old PDM, it is recommended to
- not charge the PDM more than 85%,
- and not charge it at night, in full sunlight or at >30° Celsius.
3. SENSEONICS
The following news comes from the investor call of 11/8/2022 (3Q22).
Click here for extensive press release & audio.
*Update Gemini project
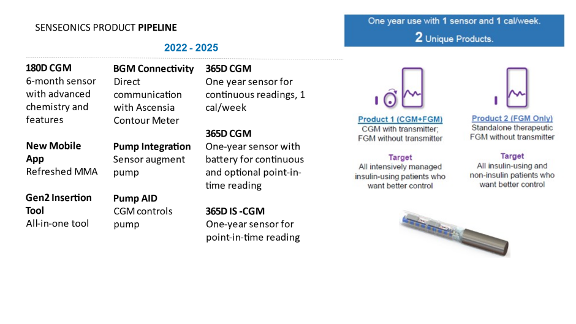
Sensionics' pipeline now looks like this:
- Eversense E3 was approved by the FDA and Europe this year. It only needs to be calibrated 1x/d from day 21. MARD is 8.5%.
- The 365-day Eversense is said to have a MARD of 9.8%. This is the same sensor as the Eversense E3, but can be left on for 365 days instead of 180 days.
- In the final step, they want to implant the battery along with the sensor. This is called the Gemini project ("Freedom system" was no longer mentioned). This system is rather a flash glucose monitoring system. To use it as a CGM, you still need the external transmitter.
4. DEXCOM
The following news comes from the investor call of 9/30/2022 (3Q22).
Click here for extensive press release, powerpoint & audio.
*Dexcom G7 resubmitted for FDA label
After the FDA's refusal this summer, Dexcom G7 has since been resubmitted. FDA approval may be expected this year.
This is important, because only then can work be done on the integration of Dexcom G7 in Tandem Control IQ and Omnipod 5.
*Dexcom ONE will also evolve to Dexcom G7 format
Dexcom will keep 2 types of sensors in its offer:
- the "more advanced" Dexcom G6/G7 for integration with insulin pumps,
- and the "simpler and cheaper" Dexcom ONE without pump integration.
This Dexcom ONE would also receive the same update as the Dexcom G7 in the future (so 60% smaller and only 30 min warm-up time).
*Dexcom sees opportunity to grow
It was already clear from the recent studies that happened and are planned, and you can see it in their presentation:
In addition to people with DM1, Dexcom also wants to reach people with DM2, and also
- people with prediabetes,
- women with gestational diabetes,
- admitted patients
- and even people without diabetes (wellness sector).
#CGMforall so...
5. ABBOTT
The following news comes from the investor call of 9/30/2022 (3Q22).
Click here for extensive press release, infographic & audio.
*Abbott is and will remain the largest
“We believe this is a $10 billion franchise by 2028.”
Mr Ford - CEO Abbott
Abbott is the largest producer of glycemic sensors, and their Libre sensor is the most widely used sensor worldwide.
Currently, 4.5 million people use a Libresensor, compared to 0.5 million 5 years ago.
Abbott shows the same ambition as Dexcom, predicting that within a few years 15 to 20 million people will use a Libre sensor, mainly due to increasing use in:
- people with DM2 who only use basal insulin,
- people with DM2 without insulin,
- and people using an insulin pump (due to their integration into closed-loop systems).

*Lingo will launch in 2023
"I think this technology will move into wellness, helping people slow pre-diabetes and, in some cases, prevent diabetes all together. We will treat for health, rather than treat for disease.”
Jared Watkin - SVP Abbott
Abbott has an extensive portfolio of biosensors:
- the Libre sensors (Libre1, Libre2 and Libre3 which would be sold at "the same price"),
- a combined ketone and glucose monitor under development
- and the Lingo system.
The Lingo sensors have the same shape as the Libre1/2 sensors and can measure glucose, ketones, lactate and alcohol (1 metabolite per sensor).
Lingo would be available from 2023. These sensors will be sold directly to users, not via the healthcare provider or healthcare provider.
*Novopen 6 integrated into LibreLink and LibreView

On 11-10-2022 there was an additional long-awaited press release: the insulin data from the Novopen 6 is available in LibreLink and LibreView.
This means that the smart Novopen 6 can really be of significance because most people on basal-bolus insulin now naturally wear a Libre sensor.
- Care providers will now finally have insight into whether, when and how much insulin was given, and can therefore provide targeted education and titrate the insulin safely.
- People with diabetes who use the Novopen 6 no longer have to enter their given insulin in their app.
These are of course only small steps, but the integration works very smoothly.
Please note: to link the Novopen 6 it may be necessary to update your LibreLink app.
*Ypsomed with Libre3 has been launched in Germany

Through this press release we learned that the mylife Loop system with a Ypso pump and Libre3 is already available in Germany!
Other countries will follow in 2023.
We are very excited about this, because this is the first closed-loop system with the Libre3 sensor - the smallest glycemic sensor worldwide.
The Libre3 sensor is also significantly cheaper than the Dexcom G6, which is certainly important for people with DM1 in Belgium.
(This technology will thus become available in the common diabetes convention and not just in the GDT convention.)
The algorithm (CamAPS FX) has been approved from 1 year old and also during pregnancy.
(The Libre3 sensor is only approved from 4 years old.)
And the closed-loop system can be controlled from your mobile phone.
(For now only Android, a version for iOS will follow in the 2nd half of 2023.)
6. EOFLOW
The following news comes from the investor call of 9/30/2022 (3Q22).
Click here for the link & powerpoint.
*Eoflow update pipeline
Eoflow plans to become a global provider of insulin pumps and closed-loop systems.
- The Eopatch pump has now been launched in 5 countries.
- This insulin patch pump is similar to the Omnipod DASH, but lasts 3.5 days instead of 3 days.
- It is waterproof for 24 hours instead of 1 hour.
- And it can be controlled with a mobile phone via the Narsha app.
- In Europe it is distributed by Menarini under the name GlucoMen Day PUMP.
- An FDA filing and an extension of the indication to people with DM2 is planned.
- The planned launch date of the Eopatch X (closed-loop system) is the end of 2023.
- Eopatch X is a closed-loop system with the Eopatch pump, a Dexcom G6 and the TypeZero algorithm. This is the same algorithm as Tandem Control IQ, so we already know that.
- A study is currently underway in 100 people with type 1 diabetes in Korea. The first patient was enrolled in May 2022, and at this point 67% of the participants would have already been included.

- In 2024 we can expect the new "Eopatch 3.0" that can stay in place for no less than 7 days.
- This patch pump was announced on 11/22/2022 and is being developed for people with DM1 and DM2.
- It has a 3 ml reservoir (instead of 2 ml like most patch pumps).
- The pump will be 22% larger and 20% heavier than the current Eopatch pump (=Glucomen Day Pump).
- Eoflow has successfully completed the first tests with these pumps, and hopes to launch this pump in 2024.
- The price will be higher than the current Eopatch pump, but not twice as much, so that a total cost reduction can be achieved.
- Eoflow will also develop its own CGM to be included in the Eopancreas.
- The Eopancreas is planned for 2025 and may be the first "fully integrated" closed-loop system!
This would of course mean a very nice step to make the lives of people with diabetes easier!
So there is a lot of interesting news to expect for next year (and after that) and I look forward to sharing it all with you.
Keep an eye on your mailbox for more updates from November and let us know if you have any questions or comments (mail to info@diabetotech.com).
See you soon!
Kind regards,













