2022 is off to a great start!
Feb 06, 2022
The first weeks of 2022 were dominated by these 2 events:
- The launch of Lingo, Abbott's biosensor that will be able to measure ketones, lactate and alcohol in addition to glucose.
- And the FDA's approval of Omnipod 5, the first closed-loop system with a patch pump and phone control.
In this blog post you can read more about why these are such important breakthroughs for people with (and without) diabetes!
Get Access To Updated Diabetes Technology Courses
1️⃣ Let's talk Lingo 💿
In early January, the International Consumer Electronics Show (CES: "the most influential technology trade show" that takes place annually in Las Vegas) presented its first presentation by a healthcare company, Abbott. You can watch the talk from Robert Ford, Abbott's CEO, here.
He showed a new version of the Libresensor: Lingo. In addition to glucose, it can also measure ketones, lactate and alcohol in real time. It is intended for the general public: from elite athletes to people who are at the beginning of their sports activities. Understanding how sports, diet and lifestyle affect these metabolites in real time will help individuals "improve their health".
The ketone monitor should be available in Europe by the end of this year, and the "Libre Sense" is already available in some European countries. No details were given about when the lactate and alcohol monitor would be launched.
Although Lingo is not developed for people with diabetes, this is good news for them! The commercialization of such biowearables will bring the price down. Abbott is certainly not the only one developing combined glucose/ketone/lactate monitors, just think of Nemaura, Biolinq, Indigo, One Drop, Percusense, iGlobe etc.
- Continuous GLUCOSE monitoring is recommended here to improve your health in general, so that you can see the effect of diet, exercise, sleep, etc. on your glycemia. They call it "biohacking", and "continuous health monitoring". Companies like Supersapiens, Veri, Levels, Januari AI and Nutrisense have proven that there is indeed a demand for this. As a non-diabetic, I have also worn a Libresensor before, and it is indeed interesting to see how your glycemia fluctuates! Further research will have to show whether the glycemic fluctuations in people without diabetes are clinically relevant or not, and what they mean.
- Continuous KETONE monitoring can help people on a keto diet to stay in ketosis. In people with type 1 diabetes, ketosis can be dangerous, because it can lead to ketoacidosis, especially in the event of illness and/or insulin deficiency. Continuous ketone monitoring can prevent ketoacidosis in people with type 1 diabetes, as well as facilitate the use of SGLT2 inhibitors. SGLT2 inhibitors can improve glycemic control and slow renal decline in people with type 1 diabetes, but this indication has not been assigned due to the risk of euglycemic ketoacidosis. Aside from several frightening stories, there are a number of people with type 1 diabetes who are taking SGLT2 inhibitors off-label.
- LACTATE monitoring can help people without diabetes to improve their sports performance, by making it easier to prevent lactate formation or muscle cramps. In people with diabetes, it could help those using a closed-loop system. With a lactate monitor, the system could detect unannounced (anaerobic) sports and adapt its algorithm accordingly.
-
ALCOHOL monitoring can theoretically be used to see how much alcohol is in your body after consumption, and whether it is safe to drive or not. Of course, this also makes it much easier to check whether drivers with an alcohol history do not drink while driving. In people with diabetes it could also be added as an extra parameter for a closed-loop system. Alcohol is a risk factor for late hypoglycemia.
The continuous detection of biomarkers such as ketones, lactate and alcohol can be done relatively easily in a minimally invasive way and is evolving rapidly. For other biomarkers such as insulin, cortisol and glucagon, the essays are for the time being limited to central labs, but work is also underway on new types of sensors.
In short, the presentation of Lingo is spectacular. On the other hand, it should come as no surprise that a large company such as Abbott keeps focusing on CGM, as this market has strong growth potential!
There are currently 5-6 million CGM users (estimated at 75% Abbott, 25% Dexcom), and it is expected that within 3-5 years the majority of people using insulin (40 million people worldwide) will be using CGM. By offering monitoring of several other metabolites, Abbott maintains its unique market position.
Even for people without diabetes, these biowearables seem to be the next step in how we monitor our sports performance and nutrition. And then of course comes the question of privacy, and who will be the owner of all this data?
Fun video's about Lingo: this one from CNET, promovideo Lingo, an inside look on CES day 1 en day 2
2️⃣ Why the Omnipod 5's FDA Approval Is Disruptive
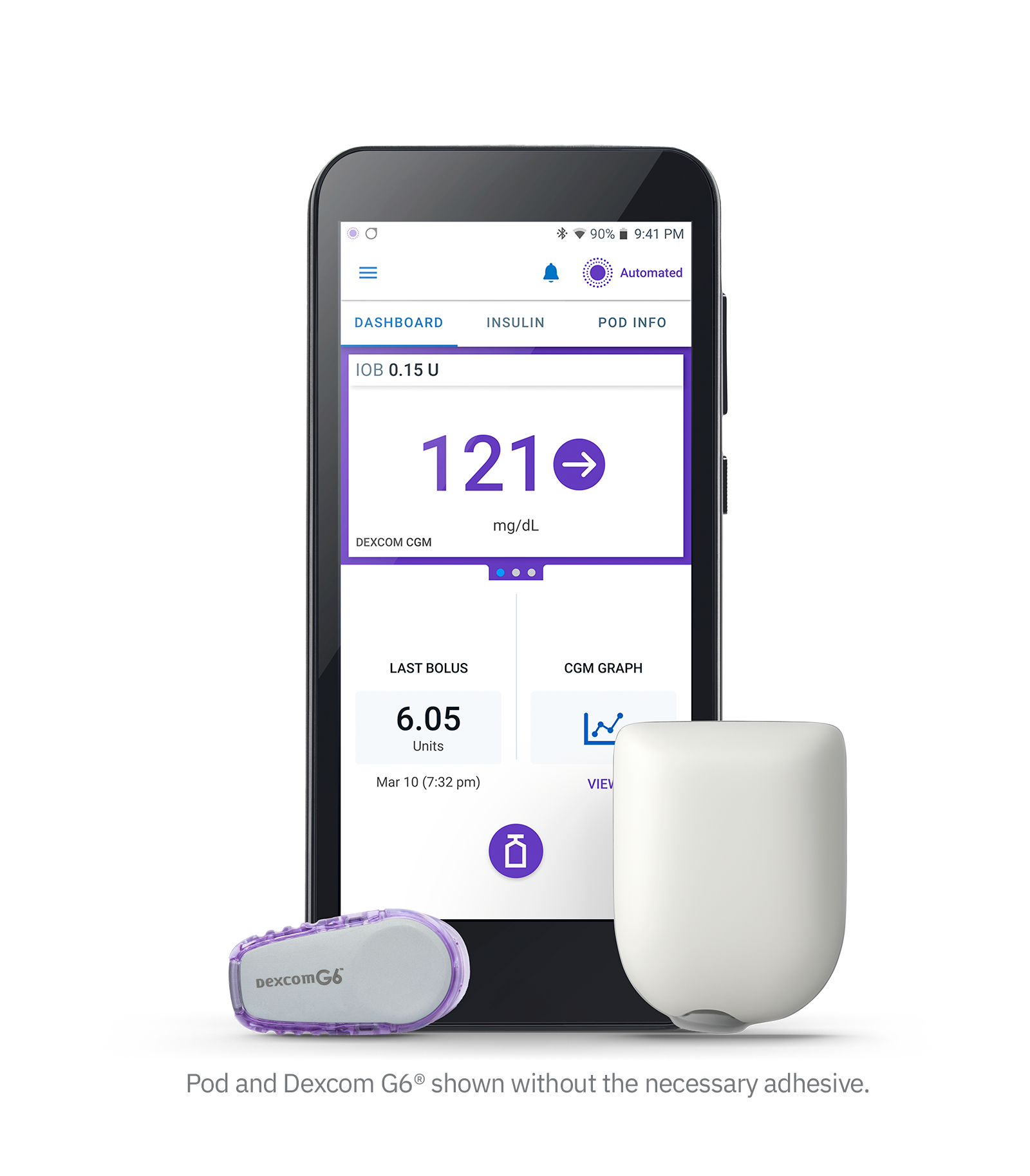
Omnipod 5 is the 3rd closed-loop system to be approved in America (alongside Control IQ and MiniMed 670G/770G), and is the first closed-loop system there with phone control and a patch pump. In America, relatively many people with type 1 diabetes use an insulin pump (around 40%) and therefore also a closed-loop system. The launch of Omnipod 5 will certainly be felt for Tandem and Medtronic!
In Europe we have a slightly more luxurious position with already 4 closed-loop systems on the market (MiniMed 670G and the much better 780G with the Guardian 4 sensor, Control IQ, Diabeloop-Insight and CamAPS FX), but Omnipod 5 would be the first closed-loop system with a patch pump (unless Kaleido-Diabeloop, Terumo-Diabeloop, Touchcare Nano System or EoPatch X comes first).
Most companies have phone control in their pipeline, but that doesn't seem to be for this year (except for Cam APS FX). Tandem requested FDA approval last year for their "phone bolus" via the t:connect app, but that isn't full phone-control. While the CEO of Diabeloop said last year that monitoring via the mobile phone would be very difficult, we now hear that they are also working on it.
Omnipod 5 will roll out in America first: limited at first, and then the rest of America after 3-9 months. They keep their lips tight when they come to Europe. A CE label may have been requested, but in any case it has not yet been approved.
Omnipod 5 consists of 3 components:
- The Omnipod patch pump that you need to replace every 3 days (looks identical to Omnipod DASH, but internally it's a new pump - the Omnipod DASH can be considered "Omnipod 4")
- The Dexcom G6 sensor that you have to replace every 10 days (connection to the Dexcom G7 will be possible "shortly after" FDA approval, for Libre they are awaiting FDA approval of the Libre3 as the Libre2 is not FDA-approved as an iCGM)
- And a new "SmartAdjust" algorithm with similar results to the MiniMed 780G and Control IQ (in children the TIR increased from 52% to 68% and in adults from 65% to 74%)
The SmartAdjust algorithm calculates the insulin dose based on sensor glycemia every 5 minutes. You can set the target glucose per time block at 110, 120, 130, 140 or 150 mg/dl. When exercising, it is best to choose the "HypoProtect mode" (a target value of 150 mg/dl and more restrictive insulin release). As with the other closed-loop systems on the market, you still have to enter your grams of carbohydrates before you eat.
Other unique features include:
- full phone control (for now only Android; iOS users can use a corresponding PDM)
- you don't have to keep a mobile phone or remote control nearby for the closed-loop system to work. The algorithm is located on the POD.
- there is no high start-up cost because you do not have to purchase an -expensive- pump. You are therefore not tied to a specific system for 4 years (cfr a catheter pump typically has a 4 year warranty).
- the bolus calculator takes into account not only the glycemia, but also the trend arrow
What after this launch?
In addition to a rollout outside the United States and integration of other glycemic sensors, Omnipod is also working on an app for iOS, and an indication for children 2-6 years and people with type 2 diabetes.
Fun videos about Omnipod 5: Nerdabetic, testimonial from an investigator and a user, promovideo Omnipod 5
And you?
Would you like to wear a glucose, ketone, lactate or alcohol sensor? And what do you hope to learn from it?
Are you waiting for a closed-loop system with a patch pump and mobile phone control? Or are you convinced of the current closed-loop systems on the market?
Kind regards,