Why people with type 1 diabetes should (not) avoid these Top 10 AID Systems
Sep 20, 2022
“Automated Insulin Delivery (AID) systems are strongly recommended for all persons with type 1 diabetes, since their use has been shown to increase Time In Range (TIR), especially in the overnight period, without causing an increased risk of hypoglycemia. Given the improvement in TIR and the reduction in hyperglycemia with AID, this method of insulin delivery is preferred above other modalities” — Grade A; High Strength of Evidence; AACE Guideline 2021
Automated Insulin Delivery (AID) systems are insulin pumps that are connected to a continuous glucose monitor (CGM) and an algorithm.
They deliver a microbolus of insulin every 5 minutes depending on the sensor glucose and a predefined glucose target.
In general, AID systems result in a better glucose control then other diabetes technologies like insulin injections or “manual” insulin pumps.
Most people that use AID systems achieve a Time In Range (TIR, that is a glucose between 70 and 180 mg/dl or 3,9 and 10,0 mmol/l) of >70%.
According to the results of the DCCT trial, a TIR of >70% will enable a person with type 1 diabetes to live a life with virtually no risk of developing long-term complications.
So although the current AID systems may be less then perfect, they will likely be good enough.
Get Access To Updated Diabetes Technology Courses
Every insulin pump manufacterer now has an AID system available or in their pipeline.
As more and more AID systems are becoming available and reimbursed, it might be hard for people with type 1 diabetes and their health care providers to know which one is the best for them.
In this article we will discuss why you should or shouldn’t choose one of these top 10 AID systems.
Insulin pump guarantee is usually 4 years, so it is important you are not stuck with a device that isn’t the best fit for you or your lifestyle.
(If there’s only one of these systems available in your diabetes center, don’t panic. The systems below are all very good AID systems which will make your life with type 1 diabetes easier.)
MiniMed 780G
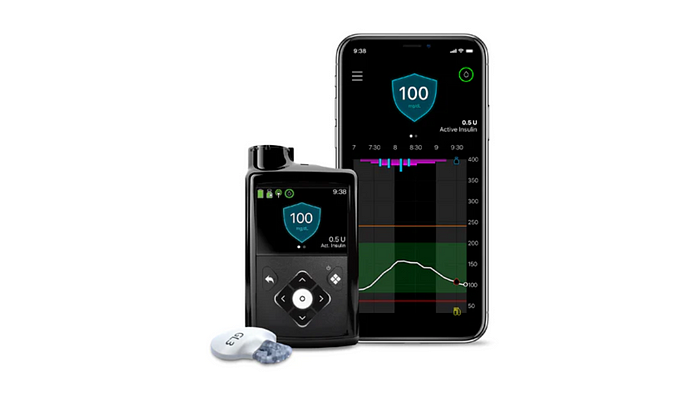
The MiniMed 780G system is broadly available in Europe, but not yet in the US.
Medtronic has applied for an FDA-label in 2020, but the approval is delayed probably due to an unresolved warning letter from the FDA in 2021.
- In the latest investor call, Mr. Martha noted that they made “substantial progress toward resolving the warning letter and preparing for reinspection,” but he did not offer a projection for how long a so-called FDA “reinspection” would take or for when 780G might be approved.
- Last week investors even sued Medtronic, because they feel Medtronic management has made misrepresentations about the MiniMed 780G being on track with FDA approval.
This means that for now, the MiniMed 780G is not available in the US, and people there are stuck with the much less advanced MiniMed 670G (or 770G).
In contrast to the misery with the FDA-approval in the US, the launch of the MiniMed 780G outside of the US is going very well.
The system is now available in over 60 countries and it is truly embraced by people with diabetes and their caregivers because of the superior results in glucose control.
The MiniMed 780G insulin pump is big and looks like a medical device
The MiniMed 780G insulin pump is one of the biggest insulin pumps on the market, and really looks like a medical device. This might seem like a minor issue, but you have to wear it all the time and it really becomes a part of you.
It is also a catheter pump, which means that the pump is connected to a katheter of 60 or 80 cm (23 or 32 inch).
- During the day you have to wear the pump in your pocket or bra, or clip it on your belt/clothes.
- During the night you have to put the pump next to you in bed.
Although it has Bleutooth, it cannot be controlled by your phone.
So you have to find a place to wear it on your body ànd be able to take out easily.
Because you need to enter the carbs that you are going to eat and respond to alarms, you will have to interact with your pump about 5–10 times a day.
Some of the Guardian 4 sensors fail before 7 days and they have the “lowest” accuracy.
Since the launch of the Guardian 4 sensor (in Europe), finger pricks are no longer needed.
This is a huge step forward coming from the Guardian 3, but there still is a problem: some of the Guardian 4 sensors are failing at 5–6 days in stead of 7 days, which will require you to change sensors unexpectedly.
Most sensors will do fine, but if you’re unlucky, you can encounter 2–3 sensors in a row that fail and make you loose a lot of time.
- Changing your sensor takes about 15 minutes as there are a lot of steps involved and you need to charge the transmitter in between.
- A new sensor needs to warm up 2 hours before it gives you glucose reedings.
The Guardian 4 sensor is also considered to be the waekest sensor in terms of accuracy.
Accuracy is usually measured in MARD (Mean Absolute Relative Difference), although it is known that this might not be the best parameter.

MARD evolution of the most used continuous glucose monitors
In general it is accepted that a CGM with a MARD of 10% or less is enough to safely dose insulin.
Despite of these issues….
the MiniMed 780G system seems to be the best commercial system out there in regards to the algorithm.
It is the only one that delivers an average TIR of >75% in real world studies.
Disclaimer: a true comparison of the different AID systems is not possible because there are no head-to-head studies available, and all studies have a different study design.
Off course, the TIR you will achieve on an AID system will also depend on the TIR you had before an AID system.
- If you had a higher TIR before the start of your AID, you will have a better result then someone who had a lower TIR at the start.
- On the other hand, if you had a lower TIR at the start of an AID, you will see a bigger increase in TIR.
The MiniMed 780G is also the only AID system that offers a 7-day infusion set for their insulin pump.
Normally you have to change your insulin infusion set or insulin patch pump every 3 days to prevent malfunction.
Reducing the number of times you need to switch infusion sets, saves you some valuable time.
Medtronic also has a very strong pipeline:
- they are actively working on eliminating carb counting,
- their next-generation CGM (Simplera) has already been submitted for CE-mark,
- and for the future they are even working on an insulin patch pump and an “infusion set and sensor in one”.
Medtronic has always been and stays the number 1 insulin pump manufacturer world wide.
They are a Fortune500 company.
Although they are making devices for more than 70 health conditions, they keep having a strong focus on diabetes.
“As a global leader in healthcare technology, the resolve to restore hope fuels our desire to strengthen, lengthen, and save lives. So we reimagine the treatment of more than 70 complex and challenging conditions. Not for the one, but for the many. Not someday, but this day.” — Medtronic
The fact that they are working in other health care areas could also have a benefit for the future of AID systems.
- They recently acquired for example exclusive distribution rights of the BioButton® multi-parameter wearable, that measures up to 1,440 vital sign measurements per day, including skin temperature, respiratory rate at rest, heart rate at rest, and more.
- And they also started to collaborate with Rockley Photonics to “deliver the next generation of wearable healthcare monitoring devices”. Rockley Photonics develops a smartwatch that measures multiple metabolites in a non invase way, such as heart rate, respiration rate, oxygen stabilization, body temperature, hydration, alcohol, lactate and glycemia.
Imagine how much more accurate the algorithm of the MiniMed 780G could become if those parameters could be incorporated…
Conclusion
So if you’re OK with some minor issues with the Guardian 4 sensor, the MiniMed 780G system seems to be a future proof AID system for people with type 1 diabetes.
Tandem Control IQ

Tandem Control IQ is the oldest AID system
Tandem Control IQ is the first AID system without the need of finger sticks that was approved in Europe and in the US.
As it is available since 2019, it is the most used AID system worldwide, with more then 250 000 people using this to make their lives with type 1 diabetes easier.
Tandem Control IQ only gets you to an average TIR of 71–74%
Because of its availability and broad use, Tandem Control IQ can show the most extensive study results.
- It was the first to publish real-world data on almost 10 000 people with type 1 and type 2 diabetes who had been using the system for more than 1 year. Their average TIR increased from 63.6% before to 73.6% after the start of the system, and it remained stable during the year-long follow-up.
- At the recent ADA congress dr. Kovatchev presented real-world data of more than 20 000 users demonstrating an overall average TIR of 71% during 3 months on the system.
Although we are not able to compare study results, we can’t help noticing that these average real world TIRs are a little lower then the ones of the MiniMed 780G system (their main compatitor).
In january 2022 a first head-to-head study between the MiniMed 780G and Control IQ system was published and concluded:
Minimed 780G appears to be more effective in managing hyperglycemia, while Tandem Control-IQ seems to be more effective in reducing time in hypoglycemia.
A disclaimer here is that this study was very small (only 90 patients) and the TIR at the start of the AID system was not the same in both groups.
We are expecting some larger head-to-head studies and we can’t wait for those results.
It’s difficult to set up the Tandem Control IQ
The Control IQ algorithm relies heavily on the parameters you enter in the pump to calculate the insulin dose.
It modulates the basal insulin dose that you enter in the pump.
So the better you know your basal insulin needs, the better the algorithm will work.
The problem is that it’s very difficult to know your basal insulin need.
There is a lot of discussion about what is the best way to set up a basal profile in an insulin pump.
Most health care providers use some form of a circadian basal profile to start with, although using a flat basal profile might be just as good (or bad).
And even if you get it right for now, it is clear that your basal insulin need will change over time.
There are a lot of circumstances which alter your insulin needs like stress, illness, temperature outside, menstrual cycle and much more.
Some people even say that estimating your basal insulin need is like chasing a ghost.
On the other hand, some people find it benificial that they are able to change more parameters that the algorithm is based on.
This gives the user more control.
In the end you know best what is going on in your life and what might be happening in the future. The ability to set different basal profiles for different circumstances can lead to a better glucose control.
The t:connect app is not coming to Europe any time soon
In the US people can use the t:connect app to bolus from their phone, although this app does not seem to be available in Europe any time soon.
In the latest investor call there was no update on the availability of this app outside of US.
This means that in Europe there is no way to see your insulin pump data on your phone, or to share that data with family members.
Even if the glucose data is available via Dexcom Share, not being able to see the insulin that has been given, could be a serious disadvantage for parents of a child with type 1 diabetes.
The same is true for the t:connect software to read out the pump data.
This designated software is only available in the US, so people in Europe will have to use Glooko to upload their pump data.
Advantages of Tandem Control IQ
Off course it’s not all negative. We actually love the Tandem pump and Dexcom sensor.
- The Tandem t:slim X2 pump looks like another phone you are carrying and has a well designed touch screen.
- And the Dexcom sensor is the most loved sensor out there because of its ease of use and its supreme accuracy.
We also like the transformative pipeline of Tandem:
- Integration with of the Dexcom G7 and Libre3 sensor is supposed to be available 6 months following FDA clearance. Mr Sheridan stated in the latest investor call that this could happen in the “early part of 2023”, although we presume it might take some more months.
- Tandem will probably also offer a seven-day infusion set in the near future, as they acquired Capillary Biomedical this summer (who is developing this).
- Tandem is working on a Mobi pump that will be a semi-patchpomp and only half the size of the Tandem pump. It will have full phone control. Submission to the FDA is expected in this quarter (22Q3).
- Next is the Tandem t:slim X3 insulin pump that will have a better bluetooth connection and a longer battery life, and a Mobi:tubeless pump that will be a full patchpump.
- The t:connect software platform will become Tandem Source, that will offer more efficient data visualisation and automatic trend recognition and advice.
Conclusion
Although their offering in the US is more advanced than in Europe, the Tandem Control IQ is a very good and well established AID system.
If you like to have more control on how the algorithm works, the possibility to change and set multiple basal profiles could be an advantage.
Omnipod 5

Omnipod 5 is not available in Europe until 2023–2024
Omnipod 5 is rolling out in the US as we speak, and has just announced a CE-label.
The first european countries are expected to launch Omnipod 5 in the middle of 2023. Because the launches will be staggered, some European countries will only be able to start Omnipod 5 in 2024.
Off course there is also a question of reimbursement, that is very different from country to country.
In the US Omnipod 5 is sold for the “at parity with” Omnipod DASH, and the controller or Personal Diabetes Manager (PDM) is free with the first prescription. The Omnipod 5 system can also be controlled by your phone with a free designated app.
The pricing outside of the US might be different. The PDM for example is not free in a lot of european countries, and the Omnipod DASH is known to be the most expensive insulin patch pump out there.
Insulet is establishing reimboursement in various european countries.
Let’s hope they find a way to get this in the hands of the people who need it the most.
The Omnipod 5 app is only available for recent Samsung phones
The Omnipod 5 system has full phone control, but only if you have a compatible phone.
For now the app is only availble for Samsung S9, S10, S20 & S21.
Insulet is working on extending the list of compatible phones, but they might need FDA-clearance for an iOS app.
That means that iPhone users will probably have to wait untill 2023–2024 before they can leave their PDM at home.
The lowest target glucose of Omnipod 5 is 110 mg/dl (6.1 mmol/l)
In the Omnipod 5 system you can only adjust your target glucose to 110, 120, 130, 140 or 150 mg/dl (6.1, 6.6, 7.2, 7.7 or 8.3 mmol/l) per time block.
So Omnipod 5 is the only AID system that doesn’t offer a glucose target lower than 110 mg/dl (or 6.1 mmol/l).
In contrast to what you might expect, this higher glucose target does not seem to have a repercussions on the TIR result that is possible with the Omnipod 5.
- If you look at their largest trial with 241 people with type 1 diabetes, the TIR in the adults increased from 64.7% to 73.9% after 3 months. These are similar results than other AID systems.
- At the ADA 2021 data were presented that showed that these TIR results sustained after 6 months.
Offcourse these are only prospective trial data. Once more people are using this system we will see the real world average TIR, which might be more relevant.
So although the Omnipod 5 has the highest target glucose, their “SmartAdjust” algorithm seems to be agressive enough to deal with hyperglycemia.
Advantages
We know that a lot of people are waiting for the Omnipod 5, because it’s their favorite AID system.
It’s the first AID system with a patch pump and it offers full phone control.
Also unique is that the algorithm is located on the pod.
- That means that even though you forget your PDM or your phone, every 5 minutes your insulin delivery will be adjusted to your sensor glucose.
- And although being under water can have an impact on the bleutooth signal, the system will work even if you’re swimming or bathing.
(Just remember: the pod is only 1 hour water resistent. So you’d better come out of the water after 1 hour.)
Insulet worked really hard to make onboarding on the Omnipod 5 as easy as possible.
They know that a lot of people in the US are trained by their general practinioner, and they are also targeting people with type 2 diabetes who normally have less basic knowledge of all this tech.
- They made easy video-based training that is freely available on their website and YouTube channel
- In the US they even offer a free 10 day trial with Omnipod 5 and Dexcom G6 so you can get to know the system before committing to it.
Another big advantage of Omnipod 5 is that they just acquired an FDA-approval for preschoolers.
That means Omnipod 5 can be prescribed (and reimbursed) for children 2–6 years old with type 1 diabetes.
We can only imagine how much this will help this fragile group of patients and their parents.
- In the US there are now 2 AID systems that are available for children 2–6 years old: the MiniMed 770G (which we didn’t discuss here as it is an older system) and the Omnipod 5.
- In Europe there is only one system that has an indication for these small children: CamAPS FX (see below).
If you pick an AID system, you’re probably going to keep using it for a few years. Although patch pumps don’t come with a guarantee of 4 years, their controller or PDM does.
So let’s check the future pipeline of Insulet:
- Integration of the Dexcom G7 sensor is expected 6 months or more after the FDA-approval of Dexcom G7. Omnipod 5 was built with Dexcom G6 in mind, and integrating Dexcom G7 will take some extra work.
- Integration of the Libre sensor will probably need a new submission to the FDA, so it’s not clear what will be the timeline on that. Insulet and Abbott are trying to realise this as fast as possible.
- Insulet is working on a next generation of Omnipod 5, although it’s not clear what they mean by this.
- Insulet is actively targeting people with type 2 diabetes. This gives them a bigger adressable market, and hopefully also the opportunity to lower their price so it can be reimbursed for more people with diabetes.
Conclusion
We understand that some people with type 1 diabetes don’t want to wear a catheter pump, and Omnipod 5 seems to be a very good alternative!
Too bad we might have to wait another 1–2 years before we can start this in Europe.
Considering the benefits of an AID system, it might not be worth it to wait that long.
Diabeloop

Diabeloop is only approved in Europe if you’re ≥18 years old
Diabeloop is a France-based software company that develops AID algorithms.
They have a CE-label for their DBLG1 and DBL-hu algorithm, but only for people ≥18 years old (excluding pregnancy).
Only the DBLG1 algorithm is commercialised.
Reimbursement is available in Germany, France, the Netherlands, Italy, Spain, Switzerland and Belgium.
In total around 7000 people with type 1 diabetes are using this system.
If you live in the US, you might have to wait a while.
Diabeloop aims to submit their DBLG1 algorithm to the FDA with the Dana pump, although the timeline is not clear.
They will first need to resume a clinical study in the US, that was suspended due to the COVID crisis. A US Scientific Advisory Board was created to help in this endeavor.
A clinical trial with their DBL4T (Diabeloop for teens 12–17 year) algorithm is ongoing, and the trial with their DBL4K (Diabeloop for kids 6–12 year) algorithm is done.
So hopefully we will see expansion of the indication of Diabeloop to people with type 1 diabetes <18 years old soon.
You will be required to carry an extra controller
Diabeloop embeds their algorithm in a controller (a locked-down Sony Xperia Z1), which you will have to keep near you everywhere you go.
If your controller is >2.5 meter (8 feet) out of range for >30 minutes, the algorithm goes into a safe basal mode.
- If you use an Accu-Check Insight insulin pump you can manually administer an insulin dose, but if you’re using the Kaleido, that is not possible.
- The Accu-Check Solo patch pump has a quick bolus button with which you can give a preprogrammed bolus amount.
Diabeloop is working on an mobile app for your phone, but it doesn’t seem to be a priority for them.
Diabeloop has difficulties finding a solid insulin pump partner
Diabeloop has agreements with several insulin pumps:
- Kaleido (ViCentra),
- AccuChek Insight and Solo (Roche),
- Dana-i (SOOIL),
- Medisafe With (Terumo)
- and even the Panda pump (SFC Fluidics).
For now, only the Diabeloop-Kaleido, Diabeloop-Insight and Diabeloop-Solo system are commercialised. The other partnerships are agreements for the future.
Although some people in France are still using the Diabeloop-Kaleido solution, most of the Kaleido users had to stop using it due to manufactering problems.
ViCentra is confident the issues are fixed and they are able to restart commercialisation of the Kaleido pump at larger scale and offer the Diabeloop-Kaleido solution in the near future.
They recently also secured funding for this.
The Diabeloop-Insight system works very well, but the Accu-Check Insight pump will not last for long.
The Diabeloop-Insight system has already been terminated in the UK and Germany and only some people in Belgium are still using it.
These people on Diabeloop-Insight will have to switch to the Diabeloop-Solo system.
The Accu-Check Solo pump has been announced in 2019, but has been postponed many times for unclear reasons.
Also now the launch of the Solo pump goes very slow.
Roche has never sold their insulin pumps in the US, and is not planning to do so. They are not really a medical device company, and primarily sell drugs and diagnostics (eg blood glucose meters and COVID tests).
Because insulin pumps are not a priority for Roche, the future of the Accu-Check insulin pumps is a bit uncertain.
The Diabeloop-Dana system is supposed to become available in the US, Europe and Korea.
It will work with the newer Dana-i insulin pump, and not with the older versions (Dana-R or Dana-RS).
This pump already has a CE- and FDA-label, but the Diabeloop-Dana system doesn’t have this approval yet.
As described below, the availability/use of the Dana-i insulin pump is still limited.
Apart from these difficulties, reimbursement of this system in France is one of the priorities of Diabeloop.
The Diabeloop-Terumo system is supposed to become available in Japan.
The Medisafe With pump from Terumo is the first and only insulin patch pump available in Japan since its commercial launch in 2018.
Although this modular insulin pump has a CE-mark, it is not commercialised in Europe yet.
In contrary to the previous big AID systems, Diabeloop is a software company and not an insulin pump manufacturer.
This makes them highly dependent on the insulin pump manufacturers that they need to collaborate with.
Diabeloop is actively working on getting solid insulin pump partners, and regularly reporting on new agreements to give confidence that they are going to able to provide an integrated AID system.
Diabeloop might not work with Dexcom G7 of Libre
Diabeloop is supposed to be interoperable device-agnostic software, but in terms of glucose sensor the options are not so big.
They only work with the Dexcom G6 for now.
They have an agreement with Percusense, but this sensor does not seem to come to market any time soon. (There are no updates for 2.5 years.)
Although Diabeloop has a very ambitious future pipeline, inclusion of Dexcom G7 or Libresensor has not been mentioned.
That’s a big difference in comparison with other AID systems:
- Tandem and Insulet have confirmed they have a working prototype of an integrated Dexcom G7 sensor in their AID systems, and are actively talking to Abbott to integrate the Libresensor also.
- Ypsomed has stated they will have a AID system with an integrated Libre3 sensor in Europe in 2023 (see below).
Let’s hope Diabeloop can secure some new glucose sensor agreement soon, and the story doesn’t end with the Dexcom G6.
Advantages
One of the big advantages of the Diabeloop algorithms is that their level of artificial intelligence/machine learning seems to be higher than any other AID system.
The algorithm learns from glucose trends of months before, in contrast to most AID-systems that only take into account glucose trends from the past few days.
Diabeloop is a totally different algorithm then the other AID’s, with some unique adjustable parameters and features.
- Besides the “activity mode” it also offers a “Zen mode” with the main purpose not to be disturbed for a period of time with hypoglycemia or alarms.
- It offers a “privacy mode” for when there is a period in time you don’t want to share your data with your health care provider
- And it recommends a certain amount of rescue carbs if necessary
In general Diabeloop gives you more control over the parameters you can change and on what the algorithm does.
Especially the agressiveness of the system can be changed in a very wide range:
- The “hyperglycemia” agressiveness can be set between 43% and 186%
- The “normoglycemia” agressiveness can be set between 59% and 147%
- And the “mealtime” agressiveness can be set between 50% and 200%
The Diabeloop system is effective in lowering HbA1c.
Although their pivotal trial data were a bit disappointing (average TIR was only 68.5%), they recently presented very good glucose control in almost 1000 real-world users in Germany (average TIR was 73%).
If you have access to the Diabeloop system, their insulin pumps have specific advantages over other insulin pumps.
- The Accu-Check Insight is a small catheter pump that works with insulin Pumpcarts. That means you don’t have to manually fill your insulin reservoir, which will save you some time.
- The Accu-Check Solo is a nice modular insulin patch pump with a quick bolus button. You can preset the amount of insulin the pump should give with every click (0.2–2 U).
- The Kaleido pump is a very small light weight semi-patch pump with the most beautiful colors, like red, orange, green, turquoise, bleu, purple, pink, black, gold or silver. Speeking of choice…
The future pipeline of Diabeloop is also very impressive:
- They are developing a full AID system (so without the need to enter sports and/or meals), with which they want to achieve a TIR of >90%! To do this, they want to include additional sensors via a smartwatch (such as heart rate variability and an accelerometer), for automatic detection of physical activity and meals.
- They are developing the DBL4pen. This is a smart insulin pen should give 80% of the results (of an AID system with an insulin pump) for 30% of the costs.
- And they are persuing more indications: for younger people (DBL4K and DBL4T), for people with unstable diabetes (DBL-hu), for all insulin types and also for people with type 2 diabetes.
Conclusion
Diabeloop is an important AID player that gives diversity and more competition to the AID market.
We love their mantra “choice matters”.
This approach fits the “personalised care” that is recommended everywhere nowadays.
CamAPS FX

CamAPS FX is only available in UK
CamDiab is a UK-based software company that offers a AID algorithms (CamAPS FX and CamAPS HX).
They secured a CE-label for their CamAPS FX algorithm, but they have not been able to succesfully expand beyond the UK.
- In the UK the CamAPS FX app is reimbursed and seems to work quite well. It is not clear how many people are currently using the system.
- There are small trials running in Luxembourg and the Netherlands, and more countries are supposed to start soon. It’s not clear when though, and if this is going to be part of a trial or not.
The CamAPS FX app is available in multiple languages: English, Czech, Danish, German, Spanish, French, Italian, Dutch, Polish, Finnish, and Swedish.
So they are well prepared for launch in more European countries.
They don’t seem to have plans for the US.
Maybe that’s because they are quite dependent on Dexcom, who might have another agenda.
Dexcom owns for example the algorithm of Tandem Control IQ, which is a very big hit in the US.
Introducing another AID system in the US market might not be in the interest of Dexcom.
You need to have your Android phone with you at all time
The CamAPS FX algorithm is not located on a locked-down handset, but is downloadable as an app on an Android phone.
- It runs on every Android model that is supported by Dexcom, so that gives you a lot of options.
- An iPhone app is not available and doesn’t seem to be planned.
You will need to have your phone with you at all time.
If your phone runs out of battery, or is are out of range, the pump will reverse to a safe basal setting.
- The maximum recommended distance between your phone and the pump is 5 to 10 meters or 16 to 30 feet.
- The maximum recommended distance between your phone and the Decom G6 is 6 meters or 20 feet.
With the Dana or Ypsopump you will be able to give an insulin bolus on the pump if necessary, even if you forgot your phone at home.
CamAPS FX users are dependent on the agreements with insulin pump partners and glucose sensors
CamAPS FX only has 2 insulin pump partners: SOOIL (Dana-RS and Dana-i pump) and Ypsomed (Ypsopump).
- The Dana-RS pump is the older version of the Dana-i and will probably not be started anymore.
- The Dana-i is a good catheter pump, although the interface of the pump feels outdated. The pump should be “more affordable” than comparable insulin pumps. It is available in Korea, Asia and New Zealand. Availability/use in Europe is limited (UK, the Netherlands and Luxembourg). It has an FDA-label, but is not used in the US yet.
- Ypsopump is a small light weight catheter pump that works with insulin Pumpcarts and has a unique infusion set that rotates 360 degrees. Ypsomed has integrated the CamAPS FX algorithm in their mylife Loop system. If you are choosing or offered an Ypsopump, its therefore more logical to choose the more integrated mylife Loop system (view below).
CamAPS FX works with Dexcom G6 and in the future with Libre3 (see also below). We didn’t here an agreement on integrating the Dexcom G7.
Advantages
There are some major advantages to the CamAPS FX system though!
- It is the only AID system that is approved for children 1–6 years old in Europe, based on the very positive results of this trial.
- It requires the lowest minimal total daily insulin dose. The algorithm is approved for a total insulin dose of ≥ 5 U, which is a little bit less than the other big AID systems: MiniMed 780G requires ≥8 U, Tandem Control IQ >10 U, Omnipod ≥6 U per day and Diabeloop >8 U.
- It is the only AID system that is approved in pregnancy, which is quite important. Especially during pregnancy you want good glycemic control, preferable with a proven safe AID system.
- And it was the first, and is still one of the only commercial AID systems to offer full phone control.
The team of CamDiab is also developing a CamAPS HX algorithm that will not require meal announcements. So this is a fully closed-loop system.
- Several studies have already been conducted, especially in hospitalized patients with type 2 diabetes (NEJM 2018, Lancet Diabetes & Endocrinology 2019, Kidney International 2019) and also in dialysis patients with type 2 diabetes (Nature Medicine 2021). A study in 30 people with type 1 diabetes will be completed by the end of this year (CLEAR study).
- Next week at EASD we are expecting the results of a trial with this system in people with type 2 diabetes on basal-bolus therapy.
Conclusion
CamAPS FX is a very good AID system, and the only AID system that is approved for pregnancy and (in Europe) for small children from 1 year old.
If this system is available for you, it’s a good choice, although you might also consider the option that follows.
Mylife Loop

Availability of mylife Loop is limited for now
Ypsomed has stopped working on their previous AID system, and is now offering the CamAPS FX algorithm in their integrated “myLife Loop” system.
It’s the same CamAPS app, but they’ve called it mylife (CamAPS) app and adjusted the colors to the Ypsomed brand. Because it’s basically the same app, they didn’t need a new CE-label.
Ypsomed offers the mylife CamAPS app free of charge to mylife YpsoPump users. Per user they pay a license fee to CamDiab.
Off course you also need reimbursement of the Dexcom G6 sensor.
The first few people in Germany, UK, the Netherlands, Switzerland and Austria are using this system already.
Scandinavia, Italy, Spain, Eastern Europe and France should be next, and more countries after that.
Ypsomed plans to submit its pump for FDA clearance in an AID system in 2023.
If cleared, Lilly will have exclusive rights to commercialize the pump in the US.
You need to have your phone with you at all time
The mylife (CamAPS) app can be downloaded on the every Android phone that is supported by Dexcom, but you will need to keep your phone with you at all time.
If your phone runs out of battery, or is are out of range, the pump will reverse to a safe basal setting.
You will be able to give a manual insulin bolus on your YpsoPump, even if you forgot your phone.
Advantages
Off course you have all the advantages of the CamAPS FX algorithm:
- it is approved for children 1–6 years old,
- it requires only ≥ 5 U as a minimal total daily insulin dose,
- it is approved in pregancy,
- and it offers full phone control.
With the advantages of the YpsoPump:
- a small lightweight insulin pump with a modern looking interface,
- works with insulin PumpCarts so you will save some time not havoing to manually fill your insulin reservoir every few days,
- and works with a unique Orbit infusion set that can rotate 360°.
YpsoMed has also 2 breakthrough innovations in their pipeline:
- They are activily working on an app for iPhone users
- And they have an agreement with Abbott to integrate the Libre3 sensor in their mylife Loop system.
This partnership is revolutionary as it will be the first AID system with a Libresensor, which is a more affordable sensor than the Dexcom G7.
The launch of the mylife Loop with Libre3 is planned for November 1st in Germany first. In 2023 other countries where Libre3 is available will follow (eg the Netherlands).
Conclusion
We love the mylife Loop system and are looking forward to their launch in the different European countries.
We also hope the mylife Loop system with the Libre3 will make AID systems more affordable and available for even more people with (type 1) diabetes.
iLet (insulin-only)

Beta Bionics keeps postponing their Bionic Pancreas
Beta Bionics has been talking about launching a dual hormone AID system with insulin and glucagon since 2015. Their “Bionic Pancreas” was supposed to be launched in 2017.
They were able to show a dual chamber insulin pump (iLet), but it appeared to be difficult to find affordable and stable glucagon.
They decided to go forward with an insulin-only iLet version, and this year the first result were finally read-out at the ATTD and ADA. Subsequently the system has been submitted to the FDA.
Although the path ahead doesn’t seem easy:
- A study with their dual hormone set (iLet Duo) was suppose to start this year, but the trial has been delayed until early 2023.
- And last month there were rumours that Beta Bionics had to lay off over 100 employees.
The iLet (insulin-only) doesn’t perform better than other AID systems
The result of the initial pivotal trial with the dual hormone “iLet Duo” in 2017 looked very promising: the average TIR increased from 61.9% to 78.4%.
If you look at the long awaited results of the insulin-only iLet pivotal trial now, it’s a bit disappointing.
The average TIR increased from 51% to 65%, which is below the recommended TIR goal of >70%, and not better than any other AID system.
Although there is one subanalysis that is quite interesting.
Apparently 31% of the people in this study were already using an AID system before they started with the iLet (Control IQ or MiniMed 670G/770G).
- These people saw a limited improvement in their HbA1c after the switch to the iLet (from 8% to 7.7%).
- 60% of these people even reported that the iLet system was better or much better than their current closed-loop system.
Advantages
Besides the seamingly moderate TIR results, the iLet is revolutionary in simplicity.
Very little interaction is required from the user or the health care provider, and all numeracy has been taken out:
- You only have to enter the body weight of the user and the closed-loop system can start (without warm-up period). It’s a self-learning algorithm and will decide by itself how much insulin it will give.
- The only thing you can adjust is the target value (usual, lower, or higher), which you can set for different times of the day.
- You don’t have to count carbs either! You only have to enter which meal you are going to eat (breakfast, lunch or dinner) and the size of that meal (usual for me, less, or more).
Conclusion
The iLet (insulin-only) is the easiest AID system out there for the user, but also for the health care provider.
You will not have to count carbs, or adjust the algorithm (as there are no parameters to adjust).
The main question is if and when this system will be available.
INREDA

The INREDA insulin pump is massive
The INREDA pump is the biggest insulin pump ever.
It’s called the AP, which is short for Artificial Pancreas.
It contains a reservoir for insulin and glucagon, but still… it’s a very big device to carry with you at all time.
- You will not be able to wear every dress, because you need to wear a belt to clip on your AP.
- INREDA can provide you a dedicated shirt with a sewed-on pocket for the AP.
- People who do a lot and/or intense sport activities are not good candidates because the AP device might fall or get damaged.
INREDA is working on a smaller device, which is especially important for children.
We haven’t seen any prototype yet, nor was any timeline for a smaller pump disclosed.
You need to wear 4 devices on your body
The INREDA AP is a catheter pump that is connected to your body with 2 infusion sets: one for the insulin and one for the glucagon.
One of their mantra’s is “one sensor is no sensor”.
That’s why their AP uses not one but 2 glucose sensors to calculate the insulin and glucagon dose.
They use 2 modified Enlite sensors, which you have to wear on the same side as you are going to carry the AP.
It’s a lot of work and you will need to prick your finger daily
Wearing the INREDA AP requires a lot more work than wearing a traditional AID system:
- You need to do 1 fingerprick a day. The INREDA AP can ask for more fingerpricks.
- You need to change your glucagon reservoir and infusion set daily. First you have to dissolve the glucagon powder in a dissolving liquid and fill the reservoir.
- Every 3 days you need to change your insulin infusion set.
- When your insulin reservoir is almost empty, you need to change it.
- Every 6 days you need to change the (AA) batteries.
- Every 7 days you need to replace 2 sensors.
- Every month you need to change the battery of the 2 transmitters.
- Every month you need to change the glucagon adaptor.
You will lose a lot of time if you want to disconnect for >30 minutes
Sometimes you will need to disconnect from to AP, for example to change your clothes, for a shower, for a swim or for sexual intercourse.
- If this is going to be less than 30 minutes, you can leave the sensors and transmitters on, and just disconnect the catheters from the infusion sets (disconnect the AP).
- When you are ready, you can just reconnect the AP without backup treatment.
Sometimes your disconnection is going to be longer, for example if you are going to the sauna, or if you need an X-ray, MRI or bodyscan, or an operation where diathermy might be used.
- In that case you do not only need to disconnect the AP (disconnect the catheters from the infusion sets), but you will also need to remove both sensors and the transmitters.
- When you are ready, you can replace the AP and the sensors, but it will take 6 hours before the sensors are warmed up and the AP can restart giving insulin and glucagon.
- Untill then, you will need to administer some backup treatment (with an insulin injection pen).
You are not allowed to take paracetamol
The INREDA AP system is based on 2 adapted Enlite sensors.
These older Medtronic sensors can give higher sensor glucose values if you are using paracetamol, which might lead to the AP giving too much insulin.
It’s stated in their manual that you cannot take paracetamol when you are using the AP…
It will be available at the earliest in 2024, only for adults with type 1 diabetes (in the Netherlands)
Robin Knoops is working on the device since 2004.
The INREDA AP system has received a CE-label since 2020 for adults with type 1 diabetes (≥18 years old), although they haven’t been succesfull in commercialising the system yet.
In the Netherlands there are currently 125 people walking around with the INREDA AP for more than 1 year.
A pivotal trial with 240 participants should start around now in 12 dutch hospitals.
After that INREDA hopes to get reimboursement of the dutch care providers/insurers so it can be available by the end of 2024 in the Netherlands.
They hope that the INREDA AP will be available in other European countries within the next 5 years.
Reimboursement might be another problem, because glucagon is very expensive.
Advantages
Although the device was only studied in 23 adults with type 1 diabetes, the results of this small trial were spectacular: the TIR increased from 53.9 naar 86.6%!
The INREDA AP is a true full AID system and easy to use.
- It doesn’t require any interaction from the user. You don’t even have to announce meals or exercise! — This will require you to let go of control!
- There is only 1 glucose target: 100 mg/dl or 5.5 mmol/l
You only have to react to the alarms of the AP if necessary, and take rescue carbs if this is recommended.
The ease of use makes this system accessible for a diverse group of people.
Conclusion
The INREDA AP is a good system if
- you’re ready to let go of control on your diabetes
- you don’t mind wearing a big insulin pump, and 4 onbody devices (2 infusion sets and 2 sensors)
- and you don’t mind taking some time daily to change your glucagon reservoir.
While we wait for availability, we are curious about the results of their next trial that will finish in 2024.
AndroidAPS

AndroidAPS is not approved by the FDA or the European Union.
Despite increasing scientific evidence, open-source AID systems are currently not approved by the FDA or European institutions.
This means that there are legal restrictions.
Healthcare providers are not allowed to recommend these systems.
However, a recent international consensus published in the Lancet argues that people with diabetes should be able to make informed decisions about their own medical care.
Therefore, it is argued that healthcare providers should not only support open-source AID systems, but should also inform people with type 1 diabetes that these systems are available.
You have to make the AndroidAPS app yourself at your own risk
Another legal restriction arising from the fact that these systems are not approved, is that they cannot be distributed as a finished product.
If you want to start with an open-source AID system, you will have to make this yourself, and this is at your own risk.
You do not only have to build the AndroidAPS app, but also an app to collect sensor glucose data and a Nightscout account.
This puts many people off, and requires a high motivation and a high understanding of how these systems work to get started.
There is no help from customer service or healthcare provider
You cannot contact the customer service of your insulin pump or glucose sensor manufacterer and ask help for your open-source AID system.
They are not allowed to offer support for these systems.
While most healthcare providers are not negative about open-source AID systems, they don’t know these systems well enough to really support you with onboarding.
A lot of parameters need to be well adjusted to your needs
On top of that you also have to set a lot of parameters, which can make the setup a bit complicated.
For example, you have to set and continuously adjust your basal insulin rate, carbohydrate ratio and insulin sensitivity if necessary.
The AndroidAPS algorithm is totally dependent on the settings you enter.
So if your settings are way off, you will not have a good glucose control with AndroidAPS, and it will be even dangerous to use it.
Before using an open-source AID system it is important to understand what your basal insulin need, carb ratio, insulin sensitivity etc is.
The available instructions are written by and for tech-savvy people
The online communities have written detailed instructions and actively support people who want to start with an open-source AID system.
However, the instructions are not easy, and it takes a long time to read everything, especially if you don’t have a background as a developer or software engineer.
And there are also almost no videos available showing how to do it.
As a result, many people with type 1 diabetes do not dare to start with AndroidAPS, and healthcare providers have a hard time guiding people who start it anyway.
Starting AndroidAPS requires a lot of tech skills, time and energy
Even if you’re willen to go through with it, you will need a lot of motivation and problem solving skills to have your AndroidAPS set up.
The average time it takes to make AndroidAPS work is 2 months.
That’s because the app requires you to complete 10 objectives, before it allows you to use the loop function.
On top of that you will need to update your app 2–3 times per year. That means: make a new app and reinstall the app on your phone.
The battery life of your phone drops drastically
Because the AndroidAPS app contuously connects to your glucose sensor and insulin pump through Bleutooth, the battery life of your phone will be much shorter.
Prepare to have a battery pack nearby at all times and/or recharge in the middle of the day.
The Bluetooth connection of your phone to your insulin pump, glucose sensor and to other devices (for example to your car/headphones) might get interrupted.
Prepare for some problem solving.
Advantages
The main advantage of open-source AID systems is that
- the software for these open-source closed-loop systems is freely available on the internet,
- and it makes more insulin pumps and glucose sensors interoperable.
It makes AID systems accessible to anyone who has access to an insulin and a glucose sensor.
(Some caution: you do need a personal computer to make the app, and an Android phone version 9.0 or higher)
AndroidAPS works with numerous insulin pumps:
- Accu-Check Combo and Insight,
- Dana-R, Dana-RS and Dana-i
- some older Medtronic pumps
- Omnipod Eros and DASH
- and DiaconnG8
and glucose sensors:
- Dexcom G4, G5 and G6
- Libre1, Libre2 (not recommended)
- Eversense
- Some Medtronic sensors
- PocTech
One of the advantages is also that there is a big community of developers that is constantly working on updating the software to the newest devices.
As we speak they are working on connecting the YpsoPump, EoPatch and Solo insulin pump, and the Dexcom ONE.
Other major advantages are:
- your AID system will have full phone control
- you can link yout glucose and pump data to your smartwatch
- you can see how the algorithm is making its calculations
- you have more control: you can adjust much more parameters
- and you will be supported by other people with type 1 diabetes who all went through setting up AndroidAPS themselves and know how important peer support is.
If you have any questions, you can drop your question in the Facebook or Discord group, and you will get an answer from your peer (developers) very quickly.
Conclusion
AndroidAPS might be a solution if you don’t have access to the AID system of your choice.
It takes a lot of motivation to start, but you will have the help of a motivated community of peers.
(Tidepool) Loop

Loop is not approved by the FDA or the European Union.
Loop is an open-source AID system, equivalent to AndroidAPS, but for iPhone users.
In contrast to AndroidAPS, Loop has been submitted to the FDA in 2020 by Tidepool, but there is no news on FDA-clearance yet.
So because of legal restrictions:
- healthcare providers are not allowed to recommend these systems.
- and you will have to make the Loop app yourself at your own risk.
There is no help from customer service or healthcare provider
You cannot contact the customer service of your insulin pump/glucose sensor manufacterer, and most healthcare providers don’t know these systems well enough to really support you with onboarding.
There is a big online Loop community that made detailed instructions and really go out of their way to help anyone who is interested in starting Loop.
The start of Loop will take a lot less time than AndroidAPS, but you still need to be tech-savvy and have good problem solving skills.
Loop offers less interoperability and requires a Link device
The Loop app can only connect to 2 insulin pumps and requires a link device (RileyLink) to do so:
- some older Medtronic pumps
- and the Omnipod Eros pump.
The Loop developers are currently testing the connection with the Omnipod DASH pump, and that one will work without a link device.
Concerning glucose sensors, the Loop app can get sensor glucose data from:
- Dexcom G4, G5 or G6
- and Medtronic sensors that are connected to a Loop-compatible Medtronic pump
So there are less options for the iPhone users, but the most popular one (Omnipod DASH) seems to be coming soon.
Advantages
The main advantage of the Loop open-source AID system is that
- the software is freely available on the internet,
- your AID system will have full phone control,
- you can link your glucose and pump data to your Apple watch,
- you can see how the algorithm is making its calculations,
- you have more control: you can adjust much more parameters,
- and you will be supported by by a big online community of peers and developers.
Conclusion
Loop might be a solution if you don’t have access to the AID system of your choice, and you are an avid iPhone user.
It takes a lot of motivation to start, but you will have the help of a motivated community of peers.
Ready to pick an AID system?
In this article we described the most important pros and cons of the 10 best AID systems out there.
We’ve also developed a visual overview of these 10 best AID systems.
This cheat sheet will reduce paralysis-by-analysis and help you see which AID system would be a good fit for you or your patient with type 1 diabetes.
Get the cheat sheet here!
We wish you all the best in this exciting journey to better glucose control!




